The Effect of Botanical Tinctures and Essential Oils on the Growth and Morphogenesis of Candida albicans
Ferreira V, Dickerson W, Farr D, Barraza M, Banker R, Dutton J, Cervigni M, Willhauck M, Tifre L, Mayman S, Stone S, Bushman S, Hull G, Morgan R, Hoover T, Mittman A, Sparks L, Smithers J, Miles D, Calvert D and Langland J
DOI10.21767/2472-0151.100012
Ferreira V1, Dickerson W1, Farr D1, Barraza M1, Banker R1, Dutton J1, Cervigni M1, Willhauck M1, Tifre L1, Mayman S1, Stone S1, Bushman S1, Hull G1, Morgan R1, Hoover T1, Mittman A1, Sparks L1, Smithers J1, Miles D1, Calvert D3 and Langland J1,2*
1Southwest College of Naturopathic Medicine, Tempe, AZ 85282, USA
2Biodesign Institute, Arizona State University, Tempe, AZ 85287, USA
3Enerpathic Technologies, Tempe, AZ 85282, USA
- *Corresponding Author:
- Langland J
Southwest College of Naturopathic Medicine, Tempe, AZ 85282, USA
E-mail: j.langland@scnm.edu
Received date: January 02, 2016; Accepted date: March 29, 2016; Published date: March 31, 2016
Citation: Ferreira V, Dickerson W, Farr D, et al. The Effect of Botanical Tinctures and Essential Oils on the Growth and Morphogenesis of Candida albicans. Herb Med. 2016, 2:1. doi: 10.21767/2472-0151.100012
Abstract
Objective: Candida albicans is an opportunistic and polymorphic fungal pathogen that affects mucosal membranes and squamous epithelia as well as being part of the normal human flora. Historically, there have been many botanical-based remedies used to treat fungal conditions, including C. albicans. This study examined the efficacy of both botanical tinctures and essential oils on the growth and morphological differentiation of C. albicans.
Methods: The in vitro growth and differentiation of C. albicans was monitored following treatment with ethanol-based tinctures and essential oils prepared from several commonly used botanicals.
Results: Results demonstrated that all ethanol-based botanical tinctures tested did not inhibit the growth of C. albicans, but several tinctures, including Marsdenia condurango, Juglans nigra (Black walnut), Anemopsis californica (Yerba mansa) and Piper cubeba (Cubeb berry), significantly reduced the morphological differentiation of the yeast into the invasive hyphae form. Alternatively, several botanical essential oils, including those from Thymus vulgaris (Thyme), Rosmarinus officinalis (Rosemary) and Cymbopogon citratus (Lemon grass) had a dramatic effect on inhibiting the growth of C. albicans.
Conclusions: These results suggest that botanical tinctures commonly used in the treatment of C. albicans infections may act by blocking the differentiation of the yeast into a more virulent hyphal form while not affecting the growth rate. In comparison, therapeutic essential oils may target both the differentiation and growth rate of C. albicans. The results support that different active constituents are present in botanical tinctures as compared to oils thereby contributing to our understanding of how these botanicals may be effective therapeutics in the treatment of C. albicans infections.
Keywords
Candida albicans; Fungi; Yeast; Hyphae; Virulence; Botanical; Tincture; Essential oils
Introduction
Candida albicans is a commensal organism in the human cutaneous and mucosal flora, but can become a major human fungal pathogen given the proper conditions allowing for hyphal differentiation, biofilm formation, and overgrowth. Some of the most common areas for candidiasis include the oral and gastrointestinal tract, vaginal canal and topically on the skin [1]. In patients experiencing vulvovaginal candidiasis, common signs include burning, itching and redness. This condition affects up to 75% of women at least once in their lifetime [2]. Skin infections are common in burn victims and neonates due to their depressed or underdeveloped immune system [2]. In the AIDS patient population, there is an increased likelihood of developing oral and esophageal candidiasis, due to decreased immune function [2]. In the general population, Candida overgrowth in the gastrointestinal tract is exacerbated by high-sugar diets or medications including steroids and antibiotics. In these cases, people will present with digestive discomfort, gas or bloating. More seriously, C. albicans can become systemic and cause an infection of the bloodstream known as candida emia. This infection can then further spread into organs, causing conditions such as hepatosplenic candidiasis or Candida peritonitis [3]. C. albicans has become the fourthleading cause of hospital-acquired blood infections in the world and mortality rates for the more severe infections remain high at about 50% [1,4-6].
During growth, C. albicans may undergo a reversible morphological transition from single-celled yeast; round budding spores (also known as blastospores), to filamentous pseudohyphae and finally, multicellular, elongated tubal cells attached end-to-end known as true hyphae [1,2,6]. This transition, which is induced upon exposure of C. albicans to a number of host conditions, is required for increased virulence. The most common host conditions required for differentiation include the presence of serum, the environmental temperature, and pH levels [2]. In studying mucosal infections, the hyphal form of C. albicans has been found embedded and overgrown in tissue leading to the conclusion that in its multicellular form is when C. albicans is at its most invasive and infectious stage [7].
Currently, there are a large variety of anti-Candida treatments dependent on the anatomical location of the yeast overgrowth. Pharmacological oral agents such as fluconazole and amphotericin B have been used in cases of internal or invasive Candida overgrowth [8]. Topical agents such as nystatin and clotrimazole have been used to treat cutaneous candidiasis as well as certain oral candida overgrowths. Miconazole and tioconazole are more commonly used topically in the vaginal canal for treatment of vaginal yeast infections [3,8]. Due to Candida being a eukaryotic pathogen, many of the antifungal agents available interact negatively with human cells. These anti-fungal treatments have many adverse effects including hepatotoxicity as the most common and concerning [2]. Invasive and systemic forms of candidiasis are more difficult to treat using these medications because of their high side effect profile, particularly in patients who are already immunocompromised [3].
In this study, we examined the efficacy of botanical tinctures and essential oils to inhibit both the growth and morphological transformation of C. albicans.
Materials and Methods
Botanical tincture and essential oil preparation
Plant material was obtained from reputable sources with documentation of authenticity. All plant material was subsequently verified by qualified botanical specialists using herbal pharmacopoeia monographs and reference keys. A voucher specimen of all plant material was deposited in a repository. For tincture preparation, the dried botanicals were ground to a fine powder, resuspended in extraction solution and incubated for 3 days at room temperature. The extract was centrifuged at 3000 × g for 10 min to remove cell debris and the extraction solution filtered through a 0.2 μM filter. For standardization and comparison, all botanical extracts had an average non-volatile constituent concentration averaging 32.1 mg/ml (ranging from 22.4 - 37.2 mg/ml). This concentration (mg/ml) is based on the weight of non-volatile constituents present in the extract per ml of aqueous liquid. The source, extraction solution (percent ethanol:water), and plant dry to extraction solution ratio for each of the botanicals used in this study is shown in Table 1. The essential oils used in this study were prepared by steam distillation or cold pressed extraction. The source and preparation method for each essential oil used in this study is shown in Table 2.
| Botanical name | Common name | Part | Origin | Company source | Extraction Soln (Ethanol:Water) |
Extract ratio (Dry:liquid) |
|---|---|---|---|---|---|---|
| Marsdenia condurango | Condurango | Vine | Equador | Raintree Health | 45%:55% | 1:08 |
| Cinnamomum zeylanicum | Cinnamon | Bark | United States | Starwest Botanicals | 65%:35% | 1:08 |
| Juglans nigra | Black Walnut | Hull | United States | Starwest Botanicals | 40%:60% | 1:08 |
| Chilopsis linearis | Desert Willow | Leaf | Sri Lanka | Starwest Botanicals | 45%:55% | 1:08 |
| Anemopsis californica | Yerba Mansa | Root | United States | Starwest Botanicals | 60%:40% | 1:08 |
| Arctium lappa | Burdock | Root | China | Starwest Botanicals | 60%:40% | 1:08 |
| Tabebuia avellanedae | Pau D'Arco | Bark | Brazil | Starwest Botanicals | 60%:40% | 1:08 |
| Alpinia officinarum | Lesser Galangal | Root | India | Starwest Botanicals | 25%:75% | 1:08 |
| Carum carvi | Caraway | Seed | Egypt | Starwest Botanicals | 60%:40% | 1:08 |
| Arctostaphylos uva ursi | UvaUrsi | Leaf | Croatia | Starwest Botanicals | 60%:40% | 1:08 |
| Nepeta cataria | Catnip | Leaf | Canada | Starwest Botanicals | 50%:50% | 1:08 |
| Allium sativum | Garlic | Bulb | China | Starwest Botanicals | 0%:100% | 1:08 |
| Cuminum cyminum | Cumin | Seed | India | Starwest Botanicals | 60%:40% | 1:08 |
| Artemisia absinthium | Wormwood | Aerial | Croatia | Starwest Botanicals | 60%:40% | 1:08 |
| Origanum vulgare | Oregano | Leaf | Turkey | Starwest Botanicals | 40%:60% | 1:08 |
| Piper cubeba | Cubeb | Berries | India | Starwest Botanicals | 60%:40% | 1:08 |
| Olea europaea | Olive | Leaf | Egypt | Starwest Botanicals | 60%:40% | 1:08 |
| Thymus vulgaris | Thyme | Leaf | Egypt | Starwest Botanicals | 0%:100% | 1:08 |
| Cymbopogon citratus | Lemon Grass | Aerial | Egypt | Starwest Botanicals | 0%:100% | 1:08 |
Table 1: The source, extraction solution (percent ethanol:water), and plant dry to extraction solution ratio for each of the botanicals.
| Botanical | Common name | Origin | Company source | Method |
|---|---|---|---|---|
| Copaifera officinalis | Copaifera | Brazil | Mountain Rose Herbs | Steam distillation of the balsam |
| Oenothera biennis | Evening Primrose | N/A | Starwest Botanicals | Cold pressed extraction of the seed |
| Cannabis sativa | Hemp | N/A | Starwest Botanicals | Cold pressed extraction of the seed |
| Thymus vulgaris | Thyme | Spain | Starwest Botanicals | Steam distillation of the leaf |
| Cymbopogon citratus | Lemon Grass | India | Esoteric Oils | Steam distillation of the leaf |
| Rosmarinus officinalis | Rosemary | N/A | Starwest Botanicals | Cold pressed extraction of the leaf |
| Melaleuca alternifolia | Tea Tree | Australia | Starwest Botanicals | Steam distillation of the leaf and aerials |
| Mentha balsamea | Peppermint | United States | Starwest Botanicals | Steam distillation of the flowering herb |
| Origanum vulgare | Oregano | Turkey | Starwest Botanicals | Steam distillation of the leaf |
| Lavandula officinalis | Lavender | India | Starwest Botanicals | Steam distillation of the flowering top |
| Cymbopogon martini | Palmarosa | India | Starwest Botanicals | Steam distillation of the grass |
Table 2: The source and preparation method for each essential oil.
Yeast growth
Media and the yeast culture Candida albicans ATCC 10231 were obtained from Hardy Diagnostics (Santa Monica, CA). For growth studies, 18-hour cultures (ranging from 3-8 × 107 colony forming units (CFU)/ml) grown at 30°C in YPD broth were diluted into media (1:1,000 dilution; yeast peptone dextrose broth (YPD)) followed by the addition of indicated concentrations of each botanical extract, essential oil, or control solution. For singlecelled yeast growth, the cultures were incubated in YPD broth at 30°C with aeration (by continuous rotation) for a maximum of 24 hours. At 0, 4, 8, and 12 hrs, samples were removed and the cell concentration determined by serial dilutions on Sabdex agar (CFU/ml media). For hyphal growth, the cultures were incubated in YPD broth with 5% fetal bovine serum at 37°C with aeration (by continuous rotation) for a maximum of 24 hours. Determination of CFU/ml was done as above. For hyphal reversal studies, the 18-hour starter cultures were grown at 37°C in YPD broth plus 5% fetal bovine serum.
Microscopy differentiation analysis
At 24 hours post-treatment, light microscopy was done to evaluate the morphological state of the C. albicans. Each broth culture was stained with Lactophenol Cotton Blue and visualized by light microscopy. A minimum of 100 cells were counted and the percent of single cells verses hyphal/pseudohyphal cells determined.
Results
In this study, several botanical tinctures and oils were utilized based on their historical and/or suggested therapeutic efficacy in the treatment of C. albicans infections. Upon testing tinctures for growth inhibitory effects on C. albicans, little or no effect was observed. Figure 1 illustrates the lack of inhibitory growth with Olea europea, Origanum vulgare, Thymus vulgaris and Cymbopogon citratus on C. albicans. Each tincture was tested at concentrations of 0 μl/ml (no herbal treatment), 10 μl/ml and 30 μl/ml. At both 37°C with serum and 30°C without serum, all of the tinctures had no significant effect on the growth of C. albicans. Similar results were observed with all other ethanol tinctures tested including Marsdenia condurango, Cinnamomum zeylanicum, Juglans nigra, Chilopsis linearis, Anemopsis californica, Arctium lappa, Tabebuia avellanedae, Alpinia officinarum, Carum carvi, Arcostaphylos uva-ursi, Nepeta cataria, Allium sativum, Cuminum cyminum and Artemisia absinthium (data not shown). Vehicle control samples treated with 60% ethanol also showed no inhibitory effect on growth (data not shown).
Figure 1 Effect of botanical tinctures on C. albicans growth rate. A starter culture of C. albicans was grown at 30°C in YPD. Diluted (1,000-fold) C. albicans cultures were left untreated (solid line), or treated with the indicated botanical extracts in the amount of 10 or 30 μl/ml at 0 hours (dashed lines). The cultures were incubated at 37°C in YPD plus serum or 30°C in YPD without serum with continuous aeration (by rotation) and CFU/ml determined every 4 hours over a period of 12 hours. Assays were repeated in triplicate.
Since morphological transformation of C. albicans to hyphal form is a key virulence factor, the herbal tinctures were tested for their ability to inhibit this transformation process. Figure 2 represents the percent hyphae formation of C. albicans cultures treated with herbal tinctures for 24 hours. As shown, when C. albicans cultures were grown at 37°C with the addition of serum, 80-100% of the culture formed hyphae, whereas when grown at 30°C without serum, typically only 10% of the culture formed hyphae with 90% as single celled form. Upon the addition of Marsdenia condurango tincture at 10 μl/ml at 37°C with serum, decreased hyphae formation was observed to about 15% of the population and further decreased to 5% of the population at 30 μl/ml. This was the strongest acting tincture upon hyphae inhibition with Piper cubeba, Juglans nigra, Anemopsis californica and Cinnamomum zeylanicum also being significantly potent with only 10-25% hyphae growth at 30 μl/ml. At 10 μl/ml these botanicals provided between 50-75% hyphal growth.
Figure 2 Effect of botanical tinctures on C. albicans morphological differentiation, A starter culture of C. albicans was grown at 30°C in YPD. Diluted (1,000-fold) C. albicans cultures were left untreated (solid bar), or treated with the indicated botanical extracts in the amount of 10 or 30 μl/ml at 0 hours (dotted bars). The cultures were incubated at 37°C in YPD plus serum or 30°C in YPD without serum with continuous aeration (by rotation) for 24 hours followed by observation by light microscopy. The percent of single cells versus hyphal/pseudohyphal cells was determined. Assays were repeated in triplicate.
Visually, Figure 3A shows microscopic images of the C. albicans grown at 0 μl/ml, 10 μl/ml and 30 μl/ml of Marsdenia condurango and Piper cubeba tinctures. At 30°C without serum at each concentration, there was mostly only single-celled yeast present for both tinctures. At 37°C plus serum without any herbal treatment large, hyphal structures were observed throughout the culture. At 10 μl/ml for both herbs, large hyphae were not detected, but pseudohyphae were present. At 30 μl/ml, the M. condurango treatment resulted with nearly only single-celled yeast being present upon observation of the broth culture. For P. cubeba at 30 μl/ml, pseudohyphae were still prevalent, but as shorter structures. These results agree with Figure 1 where the growth rate of C. albicans was not affected by the herbs, but rather the morphological state of the cells was affected.
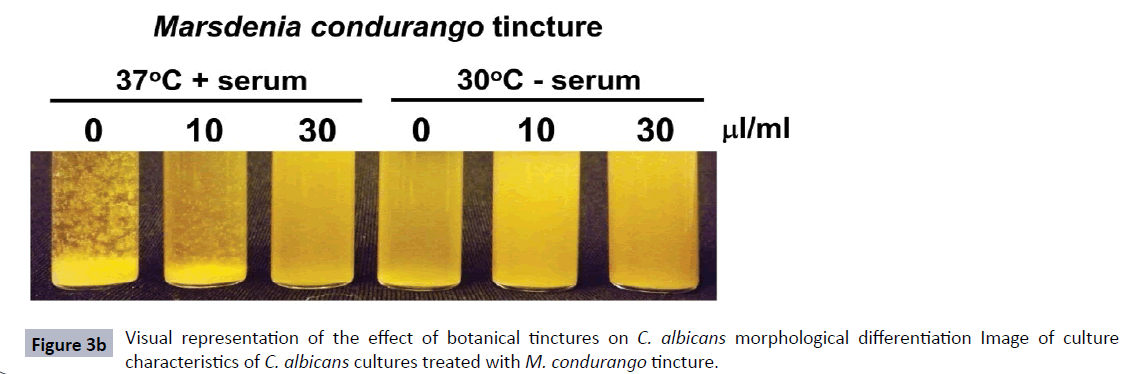
Figure 3a Visual representation of the effect of botanical tinctures on C. albicans morphological differentiation Microscopic images of C. albicans cultures treated with M. condurango or P. cubeba tinctures under conditions described in Figure 2. Examples of hyphae, pseudohyphae and single celled yeast cells are shown.
The effect of these tinctures on C. albicans differentiation can also be observed at a gross level with the broth cultures. Figure 3B shows M. condurango treatment of C. albicans cultures at 0 μl/ml, 10 μl/ml and 30 μl/ml at 37°C plus serum and 30°C without serum. At 30°C without serum, the broth cultures were turbid at every herb concentration due to the C. albicans growing in a single-celled form. When grown at 37°C with serum and no herb, the culture appeared flocculent due to large, hyphae formation (Figure 3B). This then decreased to a less flocculent and more turbid appearance at 10 μl herb/ml and a completely turbid appearance (similar to 30°C without serum) at 30 μl herb/ml.
For other herbs tested, intermediate acting herbs showed a significant effect on the morphological growth of C. albicans only at the higher doses of 30 μl/ml and were able to inhibit hyphal growth to 40-60% of the population (Figure 2). These herbs included Chilopsis linearis, Alpinia officinarum and Carum carvi. More minor effects on growth, with 75-80% of the culture still forming hyphae was seen with Olea europea, Thymus vulgaris and Cymbopogon citratus at the highest (30 μl/ml) concentrations of each tincture. Tinctures which proved to have no effect on hyphae growth were Artemisia absinthium, Arcostaphylos uva-ursi, Nepeta cataria, Allium sativum, Cuminum cyminum, Arctium lappa, Tabebuia avellanedae and Origanum vulgare (data not shown). In addition, vehicle control samples treated with 60% ethanol also showed no effect on hyphae growth or differentiation (data not shown).
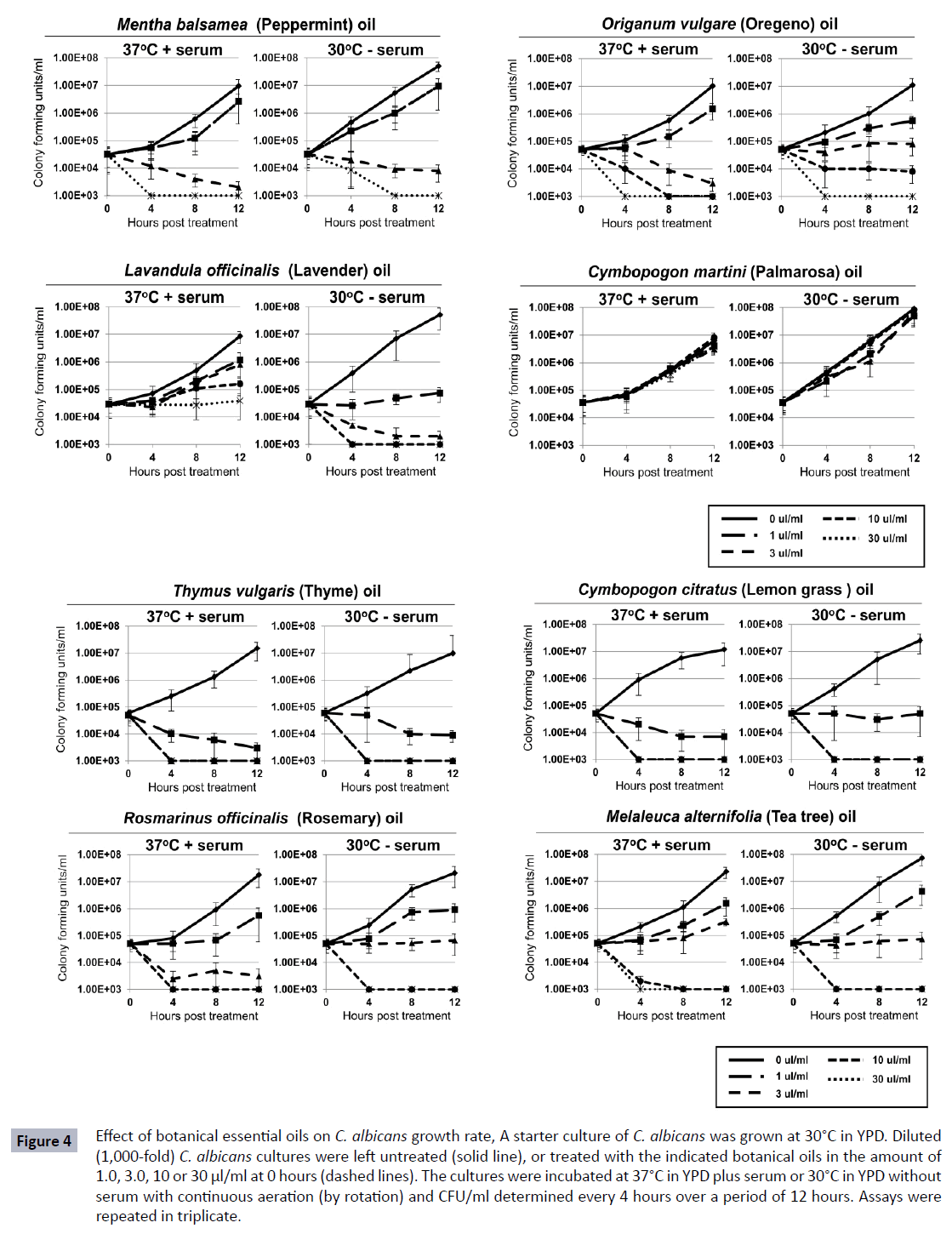
Since essential oils are also historically used in treating C. albicans infections, the effect of various essential oils on the growth and differentiation of C. albicans was also examined. Figure 4 shows that certain essential oils were able to inhibit the replication of C. albicans at both 30°C without serum and 37°C with serum. Although Thymus vulgaris and Cymbopogon citratus as tinctures showed no effect on the replication rate of C. albicans, the essential oils inhibited growth approximately 1000- fold at 1 μl/ml and over 10,000-fold at doses of 3 μl/ml or higher. This appeared to be a fungicidal effect since levels of viable cells decreased over 2-logs below the initial concentration. For other essential oils, including those from Rosmarinus officinalis, Melaleuca alternifolia, Mentha balsamea, Organum vulgare, Lavandula officinalis, and Cymbopogon martini, a more dose dependent inhibition in growth was observed (Figure 4). These herbs generally inhibited growth approximately 10-fold at 1 μl/ ml, 100-1,000-fold at 3 μl/ml, and greater than 10,000-fold at 10 μl/ml and higher. Not all essential oils tested inhibited C. albicans growth. Palmarosa oil showed no activity on the growth of the C. albicans even at the higher concentrations tested (Figure 4). Similar non-inhibitory effects were observed with essential oils from Copaifera officinalis, Oenothera biennis and Cannabis sativa (data not shown).
Figure 4 Effect of botanical essential oils on C. albicans growth rate, A starter culture of C. albicans was grown at 30°C in YPD. Diluted (1,000-fold) C. albicans cultures were left untreated (solid line), or treated with the indicated botanical oils in the amount of 1.0, 3.0, 10 or 30 μl/ml at 0 hours (dashed lines). The cultures were incubated at 37°C in YPD plus serum or 30°C in YPD without serum with continuous aeration (by rotation) and CFU/ml determined every 4 hours over a period of 12 hours. Assays were repeated in triplicate.
Since the herbal tinctures had an effect on the morphological differentiation of C. albicans, similar assays were done using the essential oils. As shown in Figure 5, Lavandula officinalis, Rosmarinus officinalis, Mentha balsamea and Melaleuca alternifolia oils were able to inhibit hyphal formation at concentrations below that which inhibited replication. For example, L. officinalis reduced hyphal formation to 5-15% at 10 and 1 μl/ml, respectively, but did not significantly affect the replication rate of C. albicans until 30 μl/ml. For Thymus vulgaris, Cymbopogon citratus and Origanum vulgare oils there was no significant effect on hyphal formation at doses that did not lead to growth inhibition (Figure 5). These results may suggest that herbs like R. officinalus, may have separate constituents which affect both growth and morphological differentiation of C. albicans, whereas herbs like T. vulgaris, only a constituent which affects only the growth rate. Cymbopogon martini essential oil showed no inhibition of hyphal differentiation at even the highest concentration of the essential oil (Figure 5). Other essential oils tested with no effect on hyphal growth include Copaifera officinalis, Oenothera biennis and Cannabis sativa (data not shown).
Figure 5 Effect of botanical essential oils on C. albicans morphological differentiation; A starter culture of C. albicans was grown at 30°C in YPD. Diluted (1,000-fold) C. albicans cultures were left untreated (solid bar), or treated with the indicated botanical oils in the amount of 1.0, 3.0, 10 or 30 μl/ml at 0 hours (dotted/dashed bars). The cultures were incubated at 37°C in YPD plus serum or 30°C in YPD without serum with continuous aeration (by rotation) for 24 hours followed by observation by light microscopy. The percent of single cells versus hyphal/pseudohyphal cells was determined. “ND” indicates samples which could not be
evaluated due to a lack of growth. Assays were repeated in triplicate.
The previous results demonstrated that several tinctures and oils could inhibit the differentiation of C. albicans from a singlecelled yeast to a hyphal form. In order to test if these herbs could also reverse differentiation from a hyphal form to a yeast form, pre-existing hyphal C. albicans was maintained under growth conditions of 37°C with serum and subsequently treated with various herbs and examined for morphological changes. These studies had the goal of indirectly assessing if an already established invasive C. albicans infection could be reversed to the less invasive and more benign state of single-celled yeast. Based on earlier results, select tinctures and oils were chosen for these assays including Marsdenia condurango and Piper cubeba tinctures and Melaleuca alternifolia, Rosmarinus officinalis, and Lavandula officinalis oils. As shown in Figure 6A and 6B, with M. condurango, the initial culture started out with approximately 90% hyphae population. At 24 hours with no treatment, the hyphal growth remained unchanged. At 10 μl tincture/ml, a significant reduction to pseudohyphae and single cells was observed at 24 hours post treatment. At 30 μl tincture/ml, hyphae formation was decreased to 95% single-celled yeast following 24 hours post-treatment. Figure 6B shows similar results for the other tinctures and oils tested with Piper cubeba tincture, Melaleuca alternifolia oil, Rosmarinus officinalis oil and Lavandula officinalis oil all significantly reversing hyphal growth to a single-celled yeast form.
Figure 6 Ability of botanical tinctures and oils to reverse C. albicans hyphal formation, A starter culture of C. albicans was grown at 37°C in YPD plus serum. Diluted (1,000-fold) C. albicans cultures were left untreated (solid bar), or treated with the indicated botanical extracts/oils in the amount of 1.0, 10 or 30 μl/ml at 0 hours (dotted bars). The cultures were incubated at 37°C in YPD plus serum or 30°C in YPD without serum with continuous aeration (by rotation) for 24 hours followed by observation by light microscopy (microscopic image shown for M. condurango in Part A). The percent of single cells versus hyphal/pseudohyphal cells was determined and graphed in Part B. Assays were repeated in triplicate.
Discussion
Historically, many botanical treatments have been used as therapeutic agents for fungal infections. In this study, botanical tinctures and essential oils were tested individually for their efficacy towards both inhibiting the growth and preventing differentiation of C. albicans. Juglans nigra has been used to treat oral infections and has been shown to have inhibitory compounds towards C. albicans [9,10]. Piper cubeba has reported constituents with bacteriostatic, fungistatic and fungicidal properties that have been found useful historically in treating oral pathogens [11-14]. Anemopsis californica has reported very strong antifungal properties making it a potentially effective topical treatment for fungal infections [15]. Some herbs, such as Marsdenia condurango are commonly used by practitioners without laboratory-based studies or evaluations. Similarly, essential oils have a long history of therapeutic use for fungal infections. Thymus vulgaris, as an essential oil, has been suggested to inhibit the growth of C. albicans intracellularly [16-18]; Rosmarinus officinalis was demonstrated to inhibit germ tube formation as an essential oil [19,20]; and Cymbopogon citratus has been suggested to inhibite mycelial formation as well as yeast bud formation [21,22].
The botanical tinctures used in this study were prepared as ethanolbased herbal extractions. Unexpectedly, based on historical use, no herbal tincture was able to inhibit the growth rate of C. albicans. However, several tinctures inhibited the morphological differentiation of C. albicans to a hyphal form. These results may suggest that therapeutic herbal tinctures may act by inhibiting the progression of single-celled yeast into the more invasive hyphae form. The strongest herbs with this mechanism of action included Marsdenia condurango, Piper cubeba, Juglans nigra, Anemopsis californica and Cinnamomum zeylanicum. C. albicans is typically a serious problem only when it differentiates into a hyphal form, therefore, it may be therapeutically valuable to limit differentiation of C. albicans instead of completely inhibiting growth and maintaining the yeast form as part of the stable flora of the microbiome in the human body.
There have been very limited previous studies done on herbs and their constituents that effectively act on inhibiting hyphal growth. Gymnemic acid is found in the herb Gymnema sylvestre and has been traditionally used in the treatment of diabetes. Similar to the herbs tested in this study, gymnemic acid did not have an effect on the growth of C. albicans, but did inhibit differentiation of the single-celled yeast to hyphae. In this case, the effective constituent was isolated and tested in hyphaeinducing conditions, which supports the future possibility of isolating novel compounds from various botanicals with which effect hyphal growth and virulence of C. albicans [5].
To further study the original goal of the experiment, which was to evaluate the fungicidal effects of herbs, essential oils were also evaluated. Essential oils are the steam-extracted, volatile aroma compounds of plants. They typically have different constituents and a higher concentration of active constituents requiring a small dose for a therapeutic effect. In this study, several essential oilbased botanicals were able to completely block hyphae formation as well as inhibiting the replication of C. albicans. Thymus vulgaris and Cymbopogon citratus were the most effective essential oils, although they had no fungicidal effect as botanical tinctures. These two herbs as tinctures only had an effect on inhibiting differentiation of C. albicans. This suggests that the same herb extracted into either oil or tincture may contain different active constituents.
Since C. albicans in patients with severe infections is commonly differentiated into the hyphal form, several tinctures and oils were also tested for their ability to reverse the hyphal form to a single-celled yeast form. M. condurango was the most effective tincture against preventing differentiation and it, along with the other active tinctures and oils were shown to be highly effective in the reversal trial. These results support the possibility for these tinctures and oils to be used in active C. albicans infections.
Tinctures are appropriate to treat both oral and topical infections, whereas essential oils are more suitable to treat topical infections due to their highly concentrated and caustic nature. Most of the botanical tinctures and oils used in this study have a low side-effect profile when taken internally or applied topically, respectively, when compared to conventional anti-fungal treatments [3]. There is a potential for both the tinctures and the oils to be combined in a synergistic blend that could be more efficacious in the overall treatment of a C. albicans overgrowth by providing different mechanisms of action.
Botanicals offer a therapeutic effect on many aspects of a fungal or microbial imbalance. Future research can explore the potential risk of antifungal resistance to these herbs, which may support the use of combined botanical preparations as opposed to a singleherb therapy and provide support for which herbs may work synergistically in order to best control C. albicans overgrowth in the human body.
Financial Support
Support for this project was provided by internal funding from the Southwest College of Naturopathic Medicine.
References
- Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJS (2013) Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options.Journal of Medical Microbiology 62: 10-24.
- Sudbery P (2011) Growth of Candida albicans hyphae. Nature Reviews Microbiology.p:8.
- Hidalgo JA, Vazquez JA (2015) Candidiasis. Medscape.
- Pfaller MA, Jones RN, Messer SA, Edmond MB, Wenzel RP (1998) National surveillance of nosocomial blood stream infection due to Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn Microbiol Infect Dis 31: 327-332.
- Vediyappan G, Dumontet V, Pelisser F, d’Enfert C (2013) Gymnemic Acids Inhibit Hypheal Growth and Virulence in Candida albicans. Plos One 8: e74189.
- Lu Y, Su C, Liu H (2014) Candida albicans hyphal initiation and elongation. Trends in Microbiology 22: 707-714.
- Mayer FL, Wilson D, Hube B (2013) Candida albicans pathogenicity mechanisms. Virulence 4: 119-128.
- Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, et al. (2004) Guidelines for Treatment of Candidaisis. Clin Infect Dis 38: 161-189.
- Noumi E,Snoussi M, Hajlaoui H, Valentin E, Bakhrouf A (2010) Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur J Clin Microbiol Infect Dis 29: 81-88.
- Alkhawajah AM (1997) Studies on the antimicrobial activity of Juglans regia. Am J Chin Med 25: 175-180.
- Silva ML, Coimbra HS, Pereira AC, Almeida VA, Lima TC, et al. (2007) Evaluation of piper cubeba extract, (-)-cubebin and its semi-synthetic derivatives against oral pathogens. Phytother Res 21: 420-422.
- Lopez A, Hudson JB, Towers GH (2001) Antiviral and antimicrobial activities of Colombian medicinal plants. J Ethnopharmacol 77: 189-196.
- Nordin MA, Wan Harun WH, Abdul Razak F (2013) An in vitro study on the anti-adherence effect of Brucea javanica and Piper betle extracts towards oral Candida. Arch Oral Biol 58: 1335-1342.
- Rath CC, Mohapatra S (2015) Suseptibility characterization of Candida spp. to four essential oils. Indian J Med Microbiol 33: 93-96.
- Yerba Mansa Monograph. India divine.
- Tullio V, Mandras N, Allizond V, Nostro A, Roana J, et al. (2012) Positive interaction of thyme (red) essential oil with human polymorphonuclear granulocytes in eradicating intracellular Candida albicans. Planta Med 78: 1633-1635.
- Rajkowska K, Kunicka-Styczynska A, Maroszynska M, Dabrowska M (2014) The effect of thyme and tea tree oils on morphology and metabolism of Candida albicans. Acta Biochem Pol 61: 305-310.
- Bogavac M, Karaman M, Janjuševic Lj, Sudji J, Radovanovic B, et al. (2015) Alternative treatment of vaginal infections – in vitroantimicrobial and toxic effects of Coriandrum sativum L. and Thymus vulgaris L. essential oils. J Appl Microbiol 119: 697-710.
- Guach LM, Silveira-Gomes F, Esteves RA, Pedrosa SS, Gurgel ES, et al. (2014) Effects of Rosmarinus officinalis essential oil on germ tube formation by Candida albicans isolated from denture wearers. Rev Soc Bras Med Trop 47: 389-391.
- Fu Y, Zu Y, Chen L, Shi X, Wang Z, et al. (2007) Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother Res 21: 989-994.
- Abe S, Sato Y, Inoue S, Ishibashi H, Maruyama N, et al. (2003) Anti-Candida albicans activity of essential oils including Lemongrass (Cymbopogon citratus) oil and its component, citral.Nihon Ishinkin Gakkai Zasshi 44: 285-291.
- Boukhatem MN, Ferhat MA, Kameli A, Saidi F, Kebir HT (2014) Lemongrass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan J Med 19: 25431.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences