Quantitative Analysis of Total Phenolic, Flavonoid Contents and HPTLC Fingerprinting for Standardization of Glycyrrhiza glabra Linn. Roots
Asif Husain, Aftab Ahmad, Mohd Mujeeb, Shah Alam Khan, Afnan Ghormulla Alghamdi,Firoz Anwar
DOI10.21767/2472-0151.10001
Asif Husain1, Aftab Ahmad2*,Mohd Mujeeb3,Shah Alam Khan4,Afnan Ghormulla Alghamdi5,Firoz Anwar6
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India
2Health Information Technology Department, Jeddah Community College, King Abdulaziz University,Jeddah, Kingdom of Saudi Arabia
3Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy,Jamia Hamdard, New Delhi, India
4Department of Pharmacy, Oman Medical College, Muscat, Sultanate of Oman
5Faculty of Medicine, Umm Al-Qura University, Makkah, Kingdom of Saudi Arabia
6Department of Biochemistry, Faculty of Science, King Abdulaziz University,Jeddah, Kingdom of Saudi Arabia
- *Corresponding Author:
- Dr. Aftab Ahmad
Health Information Technology Department, Jeddah Community College, King Abdulaziz University, PO Box 80283, Jeddah-21589, Kingdom of Saudi Arabia
Tel: +966–507243943
Fax: +966-02-2870024
E-mail: aftab786sa@hotmail.com, abdulsalam@kau.edu.sa
Received date: October 01, 2015; Accepted date: October 15, 2015; Published date: October 26, 2015
Citation: Minchen Wang. Quantitative Analysis of Total Phenolic, Flavonoid Contents and HPTLC Fingerprinting for Standardization of Glycyrrhiza glabra Linn. Roots. Herb Med. 2015, 2:1. doi: 10.21767/2472-0151.10001
Abstract
The Glycyrrhiza glabra Linn is an important medicinal plant and its roots are commonly used in various indigenous system of medicine to cure many acute and chronic diseases. The current study was designed to quantify total phenolic, flavonoid contentsin G. glabra Linn rootsto evaluate the antioxidant potential and to carry out the pharmacognostical investigations along with HPTLC fingerprinting in order to develop the quality control parameters for the standardization of this important medicinal plant. A variety of pharmacognostical investigations e.g., extractive values, total ash, water soluble ash, and acid insoluble ash, moisture content, loss on drying, pH and phytochemical screening of G. glabra Linn roots were analyzed as per the standard methods. The content of total phenolics, flavonoids, pesticide residues, aflatoxin and heavy metals were also determined as per the reported methods. The HPTLC fingerprinting of methanolic extract of the G. glabra roots was also carried out using CAMAG-HPTLC system. The results of phytochemicals investigations revealed the presence of carbohydrates, phenolic compounds, flavonoids, alkaloids, proteins, saponins, lipids, sterols and tannins in various solvent extracts. Total phenolic and flavonoid contents in methanolic extract were found to be 7.47 mg/gm and 2.25 µg/gm, respectively. Heavy metals concentrations were found to be under the standard limits. Aflatoxins and pesticides residues were not detected in the extract. The standard parameters established through this study may prove important tools for authentication, identification, purification and standardization of G. glabra Linn roots in herbal pharmaceutical industries.
Theoretical Background
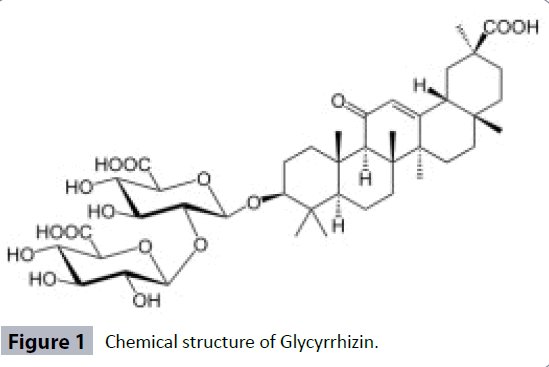
Glycyrrhiza glabra (G. glabra) Linn commonly known as Sweet wood in English is the most valuable medicinal herb that belongs to pea family ‘‘Leguminosae’’. Glycyrrhiza is derived from two Greek words “glycos” which means sweet and “rhiza” means root. G. glabra consist of unpeeled dried roots and stolon [1]. It is an important medicinal herb which is known since ancient time and it is also one of the most widely used herbs in different traditional systems of medicines worldwide [2]. It is widely cultivated in China, France, Germany, India, Italy, Russia, United Kingdom and United State of America etc. [3]. G. glabra is used as a medicine as well as a flavoring agent. The G. glabra roots contain an important active constituent called as glycyrrhizin; a saponin (Figure 1) which is reported to be 60 times sweeter than cane sugar [4]. The roots are used in traditional medicine since many centuries in the treatment of a variety of diseases in different indigenous systems of medicine including folk medicines. It is a popular remedy in traditional Ayurvedic, Chinese and Unani system of medicines [3,5]. Previously published data on G. glabra Linn revealed it to possess diverse pharmacological properties like antibacterial, antioxidant [6], anticancer [7], anti-asthmatic [8], anti-inflammatory [9], anticonvulsant [10,11], antidepressant [12], anti-hyperglycemic [13,14], hepatoprotective [15,16], immuno-modulatory [17], antimalarial [18], antiulcer [19], antiviral [20,21], analgesic [22], and memory enhancing [23]. Many more therapeutic actions of this herb are credited due to the presence of a saponin glycoside, glycyrrhizin apart from other chemical constituents [4]. Adulteration, impurities and substitution are the major problems among the natural drugs of plant origin. These problems can be overcome by using various modern analytical techniques to ensure quality control of medicinal plants and their products.
Due to the problems of adulteration, impurities and substitution, the standardization of the extract of G. glabra roots was undertaken to ensure quality of this medicinal plant. Therefore, present research work was planned to estimate total phenolic, flavonoid contents and to carry out the pharmacognostical investigations along with HPTLC fingerprinting for the standardization of the extract of G. glabra Linn Roots.
Materials and Methods
Collection and authentication of plant material
The G. glabra Linn roots were purchased from a local market of Delhi and authenticated by a taxonomist, Dr. Mohd. Mujeeb of Jamia Hamdard, New Delhi, India. A Voucher sample Specimen No: PN/FP-JNU was deposited in the Faculty of Pharmaceutical Sciences, Jodhpur National University, Rajasthan, India for future reference. The collected roots were thoroughly washed with plenty of water to get rid of the impurities and dried in the shade.
Preparation of extracts
The cleaned roots were used to prepare the extracts in different solvents. The roots were dried below 60oC in a hot air oven, powdered and passed through sieve 14 to obtain a uniform size powdered material. The dried powder (500 gm) was used for the continuous hot extraction with different solvents like petroleum ether, n-butanol, chloroform, acetone and methanol. The dried powdered material was placed in a soxhlet apparatus on a water bath for six hours. The extracts so obtained were filtered and dried with the help of rotary evaporator (Buchi, Rotavapor-R-210, Switzerland) and the final polar extract kept at low temperature for further investigations for the development of standard parameters.
Physicochemical standardization
The physicochemical standardization studies of the G. glabra roots extracts were carried out according to WHO guidelines on the quality control methods for medicinal plant materials and various other pharmacopeial procedures. A variety of physicochemical parameters like extractive values in different solvents, total ash value, water soluble ash value, acid insoluble ash value, moisture contents, loss on drying, pH values (1% and 10% solutions) were carried out for the development of the standard parameters of G. glabra root extracts. Apart from these, analysis on aflatoxins, pesticides residues and heavy metals were also carried out as per standard methods [24,25].
Preliminary qualitative phytochemicals analysis
The various solvent extracts were subjected to qualitative chemical analysis. The extracts were screened for the presence of carbohydrates, phenolic compounds, flavonoids, alkaloids, proteins, saponins, lipids, steroids and tannins [25].
Estimation of total phenolic and flavonoid contents
The total phenolic content in the methanolic extract was determined by the colorimetric assay of phenols by Folin Ciocalteu’s method. A calibration curve of standard "gallic acid" was plotted for calculation of total phenolic contents in the sample [26,27] and the total phenolic contents in the roots extract is reported as Gallic acid equivalent (GAE) (mg/g of dry mass). The total flavonoid contents of methanolic extract roots was determined by the Aluminum chloride colorimetric method using double beam UV spectrophotometer. A calibration curve of standard "Quercetin" was plotted for calculation of the total flavonoid contents in the sample [28]. The flavonoid contents in the sample of roots extract is reported as Quercetin equivalent (μg/gm of dry mass).
HPTLC fingerprinting of methanolic extract of G. glabra
A qualitative HPTLC fingerprinting analysis (CAMAG HPTLC system with a Linomat 5 sample applicator, Switzerland) of the methanolic extract was carried out for the development of characteristic fingerprint profile to confirm the presence of various phyto-pharmaceuticals. For excellent separation and sharp peaks, many solvent systems in different combinations were tried during the experiment. The solvent system of Hexane: Ethyl acetate: Methanol (9:1:1) exhibited the satisfactory resolution for the separation of phytochemicals in the methanolic extract. The whole HPTLC fingerprinting analysis was carried out in a well maintained air-conditioned room of temperature of 22°C and 55% humidity. The methanolic extract (5 μL) was spotted on the pre-coated silica gel 60F254 HPTLC aluminium plates (E-Merck, Germany) as bands of 6 mm width with the help of the auto sampler fitted with a 100 μL Hamilton syringe. The Hexane: Ethyl acetate: Methanol (9:1:1) solvent system was transferred to CAMAG twin trough plate development chamber lined with filter paper and pre-saturated with mobile phase (30 mL). The resulted plates were air dried and scanned. A spectrodensitometer (Scanner 3, CAMAG) equipped with ‘win CATS’ planar chromatography manager (version1.3.0) software was employed for the densitometry measurements, spectra recording and data processing. Absorption/remission was then measured at a scan speed of 20 mm/s. Chromatograms were recorded at 254 and 366 nm. The Rf value of each compound separated on plate and data of peak area of each band were recorded [28,29].
Determination of heavy metals, pesticide residues and Aflatoxin
The G. glabra extracts were analysed to detect the presence of heavy metals (Cd, Pb, As and Hg), pesticides residues and aflatoxins by HPLC according to official methods of the American Organization of Analytical Chemists (AOAC) [30].
Results
Physicochemical evaluations
A variety of physicochemical parameters such as extractive values, total ash, acid insoluble ash, water soluble ash, moisture content, loss on drying, pH (1% and 10% solutions) were analyzed to determine the purity of the drug. The results of the physicochemical evaluations are presented in Table 1.
| S No | Physicochemical Parameters | Average values % Mean ± SE |
|---|---|---|
| A. | Extractive Values | |
| 1. | Petroleum ether | 4.67 ± 0.23% |
| 2. | Chloroform | 10.56 ± 1.53% |
| 3 . | n-butanol, | 6.54 ±0.84% |
| 4. | Methanol | 13.89 ± 2.42% |
| B. | Ash Values | |
| 1. | Total Ash | 4.67 ± 0.35% |
| 2. | Acid Insoluble Ash | 0.56 ± 0.34% |
| 3 . | Water Soluble Ash | 6.54 ± 0.22% |
| C. | Loss on Drying (LOD) | 5.87 ± 0.65% |
| D. | Moisture contents | 0.56 ±0.054% |
| E. | pH of the extract (1% solution) | 5.04 ± 0.65 |
| F. | pH of the extract (10% solution) | 6.26 ± 0.54 |
Table 1: Results of physicochemical analysis of G. glabra Linn Roots.
Preliminary qualitative phytochemicals analysis
The results of preliminary qualitative phytochemical screening revealed the presence of alkaloids, carbohydrates, phenolic compounds, flavonoids, proteins, saponins, lipids, tannins and steroids. All mentioned phytochemicals were present in the methanolic extract of the roots. The results of the preliminary phytochemicals screening are presented in Table 2.
| Constituents | Fruits Extracts | |||
|---|---|---|---|---|
| Petroleum ether | Chloroform | n- Butanol | Methanol | |
| Alkaloids | Negative | Negative | Negative | Negative |
| Carbohydrates | Negative | Negative | Negative | Positive |
| Phenolic Compounds | Negative | Positive | Negative | Positive |
| Flavonoids | Negative | Positive | Negative | Positive |
| Proteins and Amino- Acids | Negative | Negative | Positive | Positive |
| Saponins | Negative | Negative | Negative | Positive |
| Lipids / Fats | Negative | Negative | Negative | Negative |
| Tannins | Negative | Negative | Negative | Positive |
| Sterols | Negative | Positive | Negative | Positive |
Table 2: Results of phytochemicals screening of different extracts of G. glabraLinn Roots. Positive:Present; Negative: Absent.
Total phenolic and flavonoid contents
The total phenolic contents represented as mean ± S.D in triplicate, was found to be 7.47 ± 0.05 mg/ gm of Gallic acid equivalent (GAE) in the extract. The total flavonoids contents represented as mean ± S.D in triplicate, was found to be 2.25 ± 0.03 μg/gm quercetin equivalents (QE) in the extract.
HPTLC fingerprint profile
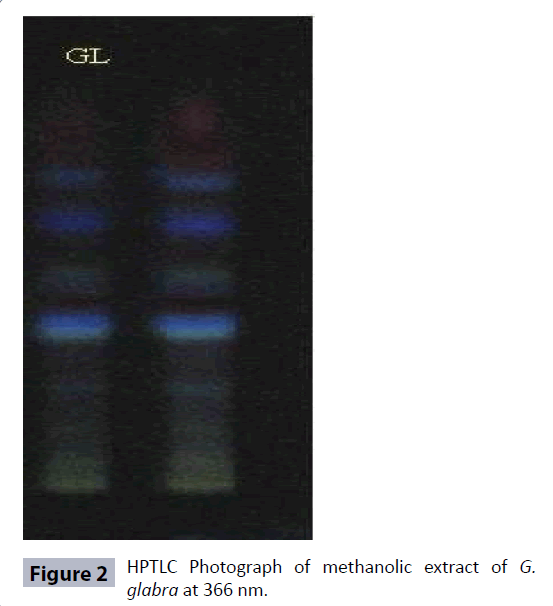
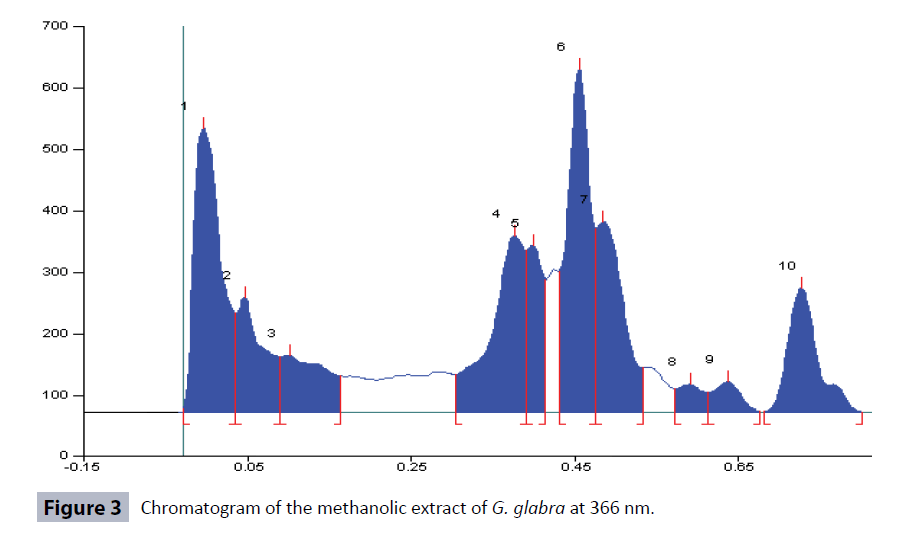
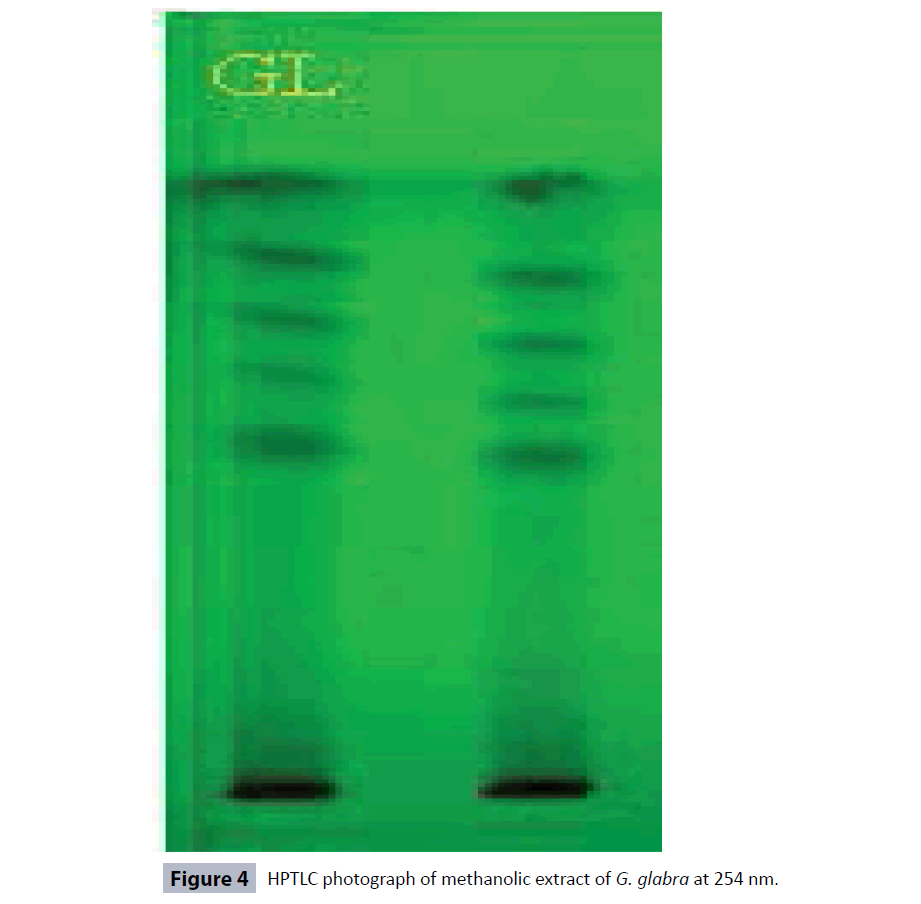
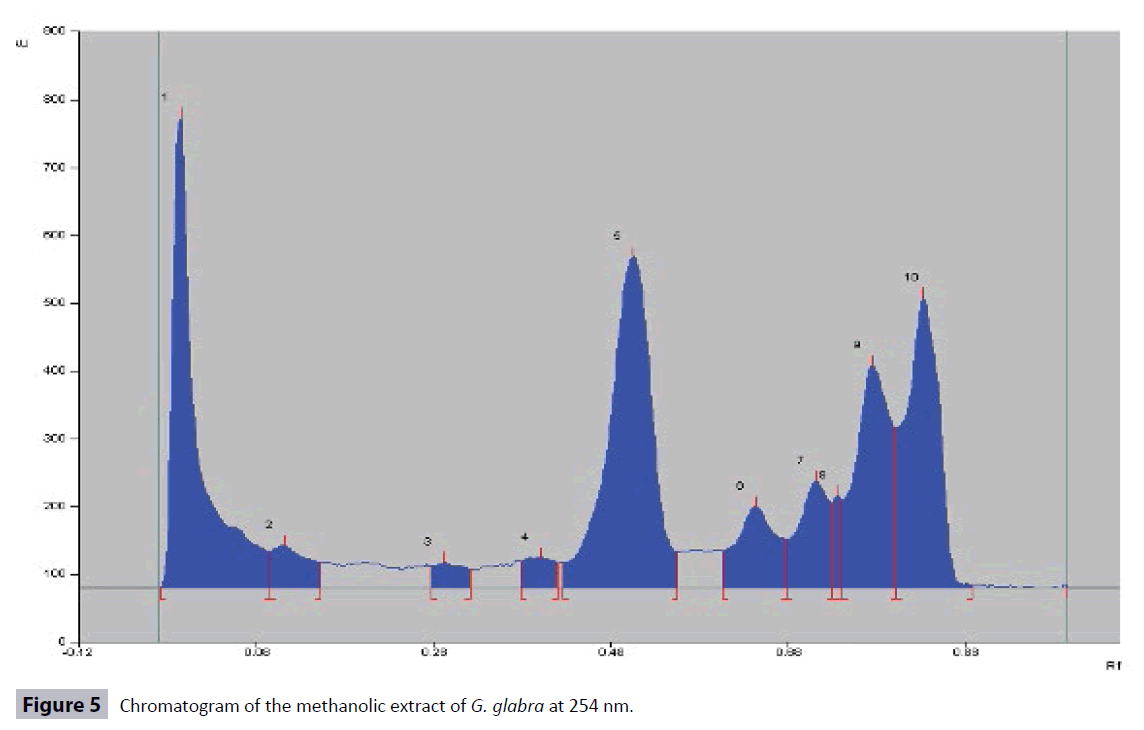
HPTLC fingerprinting of methanolic extract of the G. glabra roots was carried out by using Hexane: Ethyl acetate: Methanol (9:1:1) solvent system. A total number of 11 peaks at different Rf values and peak area at 366 nm were observed in the HPTLC chromatograms (Figures 2 and 3; Table 3) while 10 peaks were observed in HPTLC chromatogram at 254 nm (Figures 4 and 5; Table 3). The total number of phytoconstituents (no. of peaks) in the extract and their retention factors (Rf) are given in the Table 3 and chromatographic profile had been shown by Figures 2-5.
| Wave length | Solvent System | No. of peaks | Rf values | Percentage peak area |
|---|---|---|---|---|
| 366 nm | Hexane: Ethyl acetate: Methanol (9:1:1) | 11 | 0.09,0.22,0.30,0.35,0.42,0.46,0.54, 0.60,0.75,0.83,0.93 | 19.90,5.56,13.44,5.07,17.62,3.40,8.24, 2.12,17.37,4.88,2.40 |
| 256 nm | Hexane: Ethyl acetate: Methanol (9:1:1) | 10 | 0.05 ,0.19, 0.35, 0.47 0.65, 0.79, 0.92, 0.97, 0.99 ,0.93 | 2.67, 3.56, 3.47, 7.79, 6.51, 8.95, 59.28, 5.09, 4.19, 5.83 |
Table 3: HPTLC finger printing profile of methanolic extracts of G. glabraLinn Roots.
Analysis of heavy metals, pesticide residues and aflatoxins
The analysis of the heavy metals Cd, Pb, As, Hg in the extracts was carried out by atomic absorption spectrophotometer. All necessary safety precautions were taken to avoid potential contaminations. The Cadmium (Cd) concentration was found to be 0.28 ± 0.03 mg/kg which was under the permissible limit of 0.30 mg/kg according to WHO guidelines. Lead (Pb) was found to be 0.48 ± 0.12 mg/kg which was much lower than permissible limit of 10 mg/kg. Arsenic (As) and Mercury (Hg) were found to be 0.47 ± 0.05 mg/kg and 0.33 ± 0.08 mg/kg respectively in the sample of G. glabra roots extract. Both of these metals were found to be within permissible limits of 0.5 mg/kg and 1.0 mg/ kg, respectively (Table 4). A total of 40 pesticides residues were analysed in the extracts by using GC-MS. All 40 pesticides were absent in all the samples of extract. Various aflatoxins B1, B2, G1 and G2 were analyzed and none of the aflatoxins were present in any samples of the extract.
| S No | Test parameters | G. glabraLinn roots extract |
|---|---|---|
| 1 | Cadmium (Cd) | 0.28 ± 0.03 |
| 2 | Lead (Pb) | 0.48 ± 0.12 |
| 3 | Arsenic (As) | 0.47 ± 0.05 |
| 4 | Mercury (Hg) | 0.33 ± 0.08 |
Table 4: Determination of heavy metal residues (mg/kg).
Discussion
Standardization plays an important role in ensuring quality control of the herbal drugs. The quality control analysis of the medicinal plants is necessary to ensure therapeutic efficacy [31]. G. glabra is an important traditional medicinal plant used to treat wide range of ailments and diseases. Therefore, we standardized this important plant for the development of quality control parameters. A variety of physicochemical parameters were analyzed in order to standardize the G. glabra roots [28,32]. The physicochemical parameters like extractive values in different solvents, total ash value, water soluble ash value, acid insoluble ash value, moisture contents, loss on drying, pH values (1% and 10% solutions) help in ensuring the purity of the crude plant drug vis-a-vis prevents from adulteration and substitution. The extractive values are used to evaluate the presence of active constituents in the crude drug. The highest percentage yield of G. glabra roots extract was found to be 13.89% in the methanol extract (Table 1). The G. glabra roots have higher concentration of fatty constituents as revealed in the analysis. The different ash values were determined to detect the presence of any foreign matters on the surface of the drug. The total ash comprises of physiological ash and non physiological ash. The physiological ash obtained from the plant tissues of the plant while non-physiological ash is obtained from the residue of the extraneous matter adhered to the surface of the plant. The acid insoluble ash generally includes silica and hence, high acid insoluble ash is an indicator of the earthly materials contamination. The water-soluble ash indicates the quantity of inorganic elements in the sample. Therefore, Ash values plays important role for the assessment of the quality and purity of the crude drugs. The low amount of total ash, acid insoluble ash and water soluble ash indicates that absence of the impurities in the crude drug. In our results, the amount of acid insoluble ash values was found to be the lowest in the extract of the G. glabra roots (Table 1), which indicates that extract was free from earthy materials. Moisture contents and loss on drying (LOD) are other important physicochemical parameters which are used to find out the amount of moisture and volatile contents in the tested drug. The high moisture content can cause hydrolysis of the active chemical ingredients of the drug leading to low therapeutic efficacy and quality. The dryness process and rate of moisture removal play a significant role in maintaining therapeutic efficacy of the drug. The moisture contents in the extract of G. glabra roots was found to be 0.56% (Table 1), hence indicating that the drug was properly dried and well stored. Loss on drying of the G. glabra roots was found to be 5.87 ± 0.65% (Table 1). The pH of the extract of 1% and 10% solutions were also measured using digital pH meter and was found to be 5.04 ± 0.65 and 6.26 ± 0.54, respectively (Table 1). The pH of the extracts indicated the acidic nature of the compounds. The results of preliminary qualitative phytochemical screening of methanolic extract of G. glabra roots showed the presence of alkaloids, carbohydrates, flavonoids, phenolic compounds, proteins, saponins, lipids, tannins and steroids (Table 2). Hence, the current preliminary qualitative phytochemicals analysis may find application in further quantitative investigations of these important therapeutically active phytochemicals. The phenols or phenolics and flavonoids, (polyphenolic compounds) are important secondary metabolites of plants and these compounds are natural antioxidants which have wide spectrum pharmacological potentials e.g., anti-allergic, antibacterial, anticancer, anti-inflammatory, neuroprotective activities. These compounds also protect the plants from pathogenic microbial attack. Therefore, the quantitative estimation of total phenolic and flavonoid contents in the G. glabra roots was an important step to develop the quality control parameters. It is established that the roots contain significant amount of phenolic and flavonoid compounds [33]. Our study showed the presence of total phenolic contents (7.47 ± 0.05 mg/ gm) and flavonoid contents (2.25 ± 0.03 μg/gm) in the extract. The HPLTC technique is an important analytical tool for identification, detection, separation, and some other assessments of plants and their products [32]. Many phytochemicals were detected by analysing the HPTLC fingerprinting profile of methanolic extract of G. glabra HPTLC chromatogram at 366 nm showed 11 peaks at different Rf values and peak area in Hexane: Ethyl acetate: Methanol (9:1:1) solvent system (Figures 2 and 3; Table 3), while there were 10 peaks in the HPTLC chromatogram at 254 nm (Figures 4 and 5; Table 3). The number of peaks indicated the constituent's numbers in the extract and their retention factors (Rf), which are presented in Table 3 and chromatographic fingerprinting profiles have been shown in the Figures 2-5. The fingerprint images of G. glabra roots developed from these HPTLC study might be referred to as the standard reference fingerprints. These fingerprint images can be used for identification, authentication, purification, and to separate G. glabra roots from its adulterants for ensuring therapeutic efficacy. Heavy metals are highly toxic inorganic chemicals even at very low concentrations. Heavy metals stored in different parts of plants causes different toxicities in plants. Heavy metals toxicity pose a serious threat to the health of humans and animals. The most common toxic heavy metals are Cadmium, arsenic, mercury and lead. These heavy metals are present as contaminants in many herbal drugs. Exposure to lead (Pb) at higher concentration usually leads to anemia, hepatotoxicity, kidney damage, lower sperm count, miscarriage, and some neurological disorders [34]. Cadmium causes significant toxicity to many organs and systems especially on the kidney. Cadmium exposure causes anemia, cardiovascular diseases, cancers, hemorrhagic trauma, respiratory distress, rhinitis, bronchitis, and chronic obstructive pulmonary disease (COPD) [35]. Exposure to Arsenic (As) results in atherosclerosis, cancer, gastrointestinal disturbances, hypertension, liver and renal diseases, reproductive diseases, neurological disorders, skin disorders [36]. Mercury (Hg) is poisonous in all its forms. Mercury causes much toxicity like inflammation in mouth and gums, gingivitis, salivary glands, depression, dementia, muscle tremors, loss of motor coordination, severe nausea, vomiting, abdominal pain, bloody diarrhea, and kidney failure. The analysis of Arsenic, Cadmium, Lead, and Mercury concentrations in the extracts of G. glabra roots were carried out to avoid all above mentioned toxicities and standardize this valuable plant. Pesticides also cause toxicity in humans; therefore, all herbal drugs should be free from pesticides [37]. We analysed a total of 40 pesticides in the extract but none of the pesticides was positive in the tested samples. Mycotoxins are secondary metabolites of fungi. Mycotoxins may contaminate the plant and many herbal products at the time of processing and production. Aspergillus flavus, A. parasiticus, and Fusarium verticillioides are the examples of mycotoxins producing fungi. Aspergillus species are reported to produce various type of aflatoxins viz aftatoxin B1, B2, G1 and G2. All these aflatoxins are reported to cause liver cancer in humans [38]. In our study, none of these aflatoxins was present in G. glabra roots extract.
Conclusion
Herbal drugs are used worldwide for variety of therapeutic purposes. Therefore, it is very important to ensure quality of herbal drugs and herbal products. Keeping this aim in mind, the standardization of G. glabra roots extract was carried out for the development of quality control parameters of these medicinal plants which possess wide range of therapeutic potentials. We have used reliable modern techniques of pharmacognostical studies for standardization of herbal drugs. The established parameters in this study may prove important tools for authentication, identification, purification and standardization of the roots of this plant. Therefore, the generated information might be useful for preparation of monograph of this plant and might provide useful piece of information pertaining to therapeutic efficacy and quality control of this plant.
References
- Olukoga A, Donaldson D(1998) Historical perspectives on health. The history of liquorice: the plant, its extract, cultivation, and commercialisation and etymology. J R Soc Health118: 300-304.
- Obolentseva GV, Litvinenko VI, Ammosov AS(1999)Pharmacological and therapeutic properties of licorice preparations (a review). Pharm Chem J 33:24-31.
- Parvaiz M, Hussain K, Khalid S, Hussnain N, Iram N, et al. (2014) A Review: Medicinal Importance of Glycyrrhiza glabra L. (Fabaceae Family). Global J Pharmacol 8:8-13.
- Asl MN, Hosseinzadeh H (2008) Review of pharmacological effects of Glycyrrhiza sp. And its bioactive compounds. Phytother Res 22:709-724.
- Saxsena S (2005)Glycyrrhiza glabra: Medicine over the millennium. Nat prod radiance 4: 358-367.
- Varsha S, Agrawal RC, Sonam P (2013)Phytochemical screening and determination of anti-bacterial and anti-oxidant potential of Glycyrrhiza glabra root extracts. J Environ Res Develop 7:1552-1558.
- Wong TY, Lin SM, Poon CH, Leung LK (2014) The licorice flavonoid isoliquiritigenin reduces DNA-binding activity of AhR in MCF-7 cells. ChemBiol Interact 221:70-76.
- Liu B, Yang J, Wen Q, Li Y(2008)Isoliquiritigenin, a flavonoid from licorice, relaxes guinea-pig tracheal smooth muscle in vitro and in vivo: Role of cGMP/PKG pathway. Eur J Pharmacol587:257-266.
- Chen CL, Zhang DD (2014) Anti-inflammatory effects of 81 Chinese herb extracts and their correlation with the characteristics of traditional Chinese medicine. Evid Based Complement Alternat Med 2014:985176.
- Chowdhury B, Bhattamisra SK, Das MC (2013) Anti-convulsant action and amelioration of oxidative stress by glycyrrhizaglabra root extract in pentylenetetrazole- induced seizure in albino rats. Indian J Pharmacol 45:40-43.
- Nassiri-Asl M, Saroukhani S, Zamansoltani F(2007)Anticonvulsant Effects of Aqueous Extract of Glycyrrhiza glabra Root in PTZ-Induced Seizure in Mice. Int J Pharmacol 3:432-434.
- Dhingra D, Sharma A (2006) Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. ProgNeuropsychopharmacolBiol Psychiatry 30:449-454.
- Gaur R, Yadav KS, Verma RK, Yadav NP, Bhakuni RS(2014) In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine21:415-422.
- Sil R, Ray D, Chakraborti AS (2013) Glycyrrhizin ameliorates insulin resistance, hyperglycemia, dyslipidemia and oxidative stress in fructose-induced metabolic syndrome-X in rat model. Indian J ExpBiol 51:129-138.
- Li JY, Cao HY, Liu P, Cheng GH, Sun MY (2014)Glycyrrhizic acid in the treatment of liver diseases: Literature review. Biomed Res Int2014:872139.
- Rasool M, Iqbal J, Malik A, Ramzan HS, Qureshi MS, et al. (2014)Hepatoprotective Effects of Silybummarianum (Silymarin) and Glycyrrhiza glabra (Glycyrrhizin) in Combination: A possible synergy. Evid Based Complement Alternat Med2014:641597.
- Bordbar N, Karimi MH, Amirghofran Z(2012)The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cell Immunol280:44-49.
- Kalani K, Agarwal J, Alam S, Khan F, Pal A, et al. (2013) In silico and in vivo anti-malarial studies of 18β glycyrrhetinic acid from Glycyrrhiza glabra. PLoS One 8:e74761.
- Asha MK, Debraj D, Prashanth D, Edwin JR, Srikanth HS, et al. (2013) In vitro anti-helicobacter pylori activity of a flavonoid rich extract of Glycyrrhiza glabra and its probable mechanisms of action. J Ethnopharmacol 145:581-586.
- Adianti M, Aoki C, Komoto M, Deng L, Shoji I, et al. (2014) Anti-hepatitis C virus compounds obtained from Glycyrrhizauralensis and other Glycyrrhiza species. MicrobiolImmunol 58:180-187.
- SabouriGhannad M, Mohammadi A, Safiallahy S, Faradmal J, Azizi M, et al. (2014) The Effect of aqueous Extract of Glycyrrhiza glabra on Herpes Simplex Virus 1. Jundishapur J Microbiol 7:e11616.
- Shi Y, Wu D, Sun Z, Yang J, Chai H, et al. (2012) Analgesic and uterine relaxant effects of isoliquiritigenin, a flavone from Glycyrrhiza glabra. Phytother Res 26:1410-1417.
- Dhingra D, Parle M, Kulkarni SK(2004) Memory enhancing activity of Glycyrrhiza glabra in mice. J Ethnopharmacol 91:361-365.
- Anonymous(1998) AOAC (Association of Official Analytical Chemists). Wet digestion for non-volatile metals in: AOAC official methods of analysis.
- Khandelwal K (2007) Practical pharmacognosy, techniques and experiments. 17th edn, Pune, India,NiraliPrakashan Publishers.
- Lin JY, Tang CY (2007) Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem101:140-147.
- Alhakmani F, Kumar S, Khan SA (2013) Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringaoleifera. Asian Pac J Trop Biomed 3: 623-627.
- Ahmad A, Husain A, Mujeeb M, Siddiqui NA, Damanhouri ZA, et al. (2014) Physicochemical and phytochemical standardization with HPTLC fingerprinting of Nigella sativa L. Seeds. Pak J Pharm Sci27:1175-1182.
- Mauji Ram M, Abidin MZ, Khan MA,Jha P (2011) HPTLC fingerprint analysis: A quality control of authentication of herbal phytochemicals, Chapter 7, In: High-performance thin-layer chromatography(HPTLC), Berlin, Heidelberg, Springer-Verlag:105.
- Anonymous(2006) Association of official analytical chemists. In: Official methods of analysis of the AOAC (W. Horwitiz, editor) In. Washington DC.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Alhadrami HA, et al. (2015) Quantification oftotal phenol, flavonoid content and pharmacognostical evaluation including HPTLC fingerprinting for the standardization of Piper nigrumlinn fruits. Asian Pac J Trop Biomed 5: 101-107.
- Mohammed MS, Alajmi MF, Alam P, Khalid HS, Mahmoud AM, et al. (2014) Chromatographic finger print analysis of anti-inflammatory active extract fractions of aerial parts of Tribulusterrestris by HPTLC technique. Asian Pac J Trop Biomed 4:203-208.
- Jaberian H, Piri K, Nazari J (2013) Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem 136:237-244.
- Haider S, Naithani V, Barthwal J, Kakkar P (2004) Heavy metal content in some therapeutically important medicinal plants. Bull Environ ContamToxicol 72:119-127.
- Bernhoft RA (2013) Cadmium toxicity and treatment. Scientific World Journal:394652.
- Nema NK, Maity N, Sarkar BK, Mukherjee PK (2014) Determination of trace and heavy metals in some commonly used medicinal herbs in ayurveda. ToxicolInd Health 30:964-968.
- Rao MM, Kumarmeena A, Galib (2011) Detection of toxic heavy metals and pesticide residue in herbal plants which are commonly used in the herbal formulations. Environ Monit Assess 181:267-271.
- Mohd-Redzwan S, Jamaluddin R, Abd-Mutalib MS, Ahmad Z(2013)A mini review on aflatoxin exposure in malaysia: Past, present and future. Front Microbiol4: 334.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences