Positive Regulation of Rhizophora mucronata Poir Extracts on Blood Glucose and Lipid Profile in Diabetic Rats
Gurudeeban S,Satyavani Kaliamurthi and Ramanathan Thirugnanasambandam
DOI10.21767/2472-0151.100016
Gurudeeban S*, Satyavani Kaliamurthi and Ramanathan Thirugnanasambandam
Centre of Advanced Study in Marine Biology, Faculty of Marine Science, Annamalai University, Chidambaram-608 502, Tamil Nadu, India
- *Corresponding Author:
- Dr. Gurudeeban S
Centre of Advanced Study in Marine Biology
Faculty of Marine Sciences
Annamalai University, Parangipettai
Chidambaram-608 502
Tamil Nadu, India
Tel: +91 8489055578
E-mail: gurudeeb99@gmail.com
Received date: April 30, 2016; Accepted date: May 13, 2016; Published date: May 19, 2016
Citation: Gurudeeban S, Kaliamurthi S, Thirugnanasambandam R. Positive Regulation of Rhizophora mucronata Poir Extracts on Blood Glucose and Lipid Profile in Diabetic Rats. Herb Med. 2016, 2:2.
Abstract
Background: About, five percent of the population in the world has been clinically determined to have diabetes mellitus and considered as one of the principal reasons for death. The present research designed to assess the impact of Rhizophora mucronata leaves ethanolic extract (EtOH-Et) and its dichloromethane (DCM) and aqueous (Aq) fractions in diabetic rats.
Methods: Extract and fractions of R. mucronata were determined qualitative and quantitative methods. Different dose (50, 100 and 200 mg/kg of body weight) of extract and fractions were administered intraperitoneally (I.P.) to the normal glycemic rats and their hypoglycemic effect determined for 24 h. Type 1 diabetes mellitus (T1DM) was induced by single injection of streptozotocin (STZ; 60 mg/ kg; I.P.), and type 2 diabetes mellitus (T2DM) was induced by STZ (60 mg/kg; I.P.), after 15 min nicotinamide (120 mg/kg; I.P.). The optimum dose of 100 mg/kg body weight of extract and fractions was administrated to the rats orally for 14 days. The blood glucose level estimated on 0th, 7th, and 14th day. The level of serum cholesterol, triglycerides, and lipoprotein cholesterol were also evaluated on the 14th day.
Results: The phytochemical results indicated alkaloids, flavonoids, and terpenoids present in the concentrates of R. mucronata. The content of alkaloids in DCM-F was present in high amount compared with ethanolic extract and an aqueous fraction. All the extract exhibited as non-toxic nature. DCM-F treated rats significantly reduced in blood glucose level (P<0.01), serum cholesterol (P<0.05) and triglycerides (P<0.05) levels whereas HDL-C level was found to be increased (P<0.05) as compared with the diabetic control of T1DM and T2DM.
Conclusion: DCM-F of R. mucronata act as effective anti-hyperglycaemic and antihyperlipidemic agent in insulin dependent and non-insulin dependent diabetic rats.
Keywords
Acute toxicity; Diabetes mellitus; Dichloromethane; Mangrove; Total cholesterol
Introduction
Inadequate release of insulin or inconsistent action of insulin results in the prolonged level of blood glucose prompts diabetes mellitus [1]. About 2.5-7% of the population throughout the world has been diagnosed with T2DM and considered as one of the foremost causes of death [2]. Malfunction of essential body organs like eyes, heart, kidney, nerves, and blood vessels is a part of the major risk factors in the case of chronic hyperglycemia. Several studies have elaborated the factors responsible for T2DM, and the adverse effect of the clinically available drug [3]. However, the usage of medicinal plants extracts and their metabolites as antidiabetic agents still is increasing in the developing countries because of the side effects of the commercial drugs. The mangrove species R. mucronata Poir (family: Rhizophoraceae) used as a traditional medicine in the coastal villages of Cuddalore district in India [4]. Studies reported that R. mucronata extracts had a hypoglycemic, anti-radical, anti-cancer, anti-nociceptive, antiplasmodial, anti-HIV, Peroxisome proliferator activated receptor-γ antagonistic effect, α-amylase along with α-glucosidase inhibitory effect [5-11]. The fruit flour of R. mucronata was used as function food for anti-diabetic effect [12]. The alkaloid fractions of R. mucronata leaves have been reported with anti-microbial and radical scavenging effects [13]. There are a number of terpenoids, volatile derivatives, and phenolic constituents identified from the different extracts of this plant [14-16]. The computational studies expressed that alkaloids of R. mucronata efficiently interacted with dipeptidyl peptidase-4 receptor (DPP-IV) receptor, alphaketoglutarate- dependent dioxygenase, and cyclooxygenase II receptors and inhibit their mode of action [17-19]. Therefore, we hypothesized that the ethanolic extract and its fractions of R. mucronata as competent to control blood glucose, and this was evaluated in the diabetic animal model.
Materials and Methods
Chemicals and drug
Streptozotocin (STZ) and nicotinamide (NAD) were purchased from Sigma, St. Louis, USA. Colchicine, Quercetin, Gallic acid and other experimental chemicals used were of analytical grade (Hi-media, Mumbai, India). The diagnostic kits for triglycerides, total cholesterol, and high-density lipoprotein C (HDL-C) were obtained from Asritha Diatech, Hyderabad, India.
Test animals
Male, albino Wistar rats, weighing 200-250 g were procured from the Central Animal House Facility, Rajah Muthiah Medical College and Hospital (RMMC & H), Annamalai University utilized in this study. Animals were fed on a pellet diet (Hindustan Pvt. Ltd., India) and water ad libitum. The animal experiments were followed by the guidelines of National Institute of Health and approved by RMMC&H, India.
Plant material
During post monsoon season in 2010, the fresh leaves of R. mucronata were collected from the Kodiyampalayam, Nagapattinam district, Tamil Nadu, India. The plant material was deposited (Voucher no. AUCASMB11/2010) and authenticated at molecular level (GenBank Accession No.: JQ042819; JN008864).
Extraction and fractionation of R. mucronata leaves: The collected leave materials of R. mucronata (1.5 kg) were washed under running tap water, shade dried and powdered. The known amount of powdered material was extracted five times with 5×5 L of ethanol (80%) at 28°C. The solvent was removed under vacuum to get a dark greenish-brown mass of ethanolic extract (27.52 g). This extract was acidified with 1M of hydrochloric acid (HCl) and was then dissolved and fractionated with dichloromethane. Two layers, namely DCM and aqueous acid layers were obtained. The DCM layer was separated and allowed to evaporate. The sedimentary acid layer was further basified by sodium hydroxide and extracted with ethanol. EtOH-Et, DCM-F, and Aq-F were obtained. The qualitative chemical test was performed to determine the phyto-constituents present in the extract and fractions [20].
Estimation of total alkaloids: The total alkaloid content of extract and fractions of R. mucronata was estimated according to the bromocresol green complex formation method [21]. All experiments performed thrice. Colchicine used as a standard. Results were expressed as mg of colchicine equivalent per gram of dry weight (mg colchicine /g) of extracts.
Estimation of total phenols and flavonoids: Total phenols of the extract and fractions of R. mucronata were estimated using Folin-Ciocalteu’s reagent. Gallic acid used as a standard. The total phenolic content is here expressed as milligram of gallic acid equivalents (GAE) per gram of dry weight of extract [22]. Total flavonoid content was determined using aluminum chloride (AlCl3) and quercetin as a standard. The results expressed as milligram of quercetin equivalent per gram of dry weight (mg quercetin/g) of extracts [23].
Lethal dose 50 (LD50) of R. mucronata extract and fractions: Lethal dose 50 (LD50) of R. mucronata extract and fractions was performed according to the organization for economic co-operation and development (OECD)-423 guidelines [24]. Overnight fasted mice of both sexes were treated with three different doses of 50, 300, and 2000 (mg/kg b.wt.) of EtOH-Et, DCM-F, and Aq-F, through oral route respectively. The control mice received water alone. The animals were observed for first 72 h and up to seven days for any signs of behavioral changes, toxicity effect, mortality, and body weight. The number of mice died within 24 h was noted, and their lethal dose 50 (LD50) of the extract and fraction was calculated.
Administration of extracts and fractions to normal rats for hypoglycemic studies: The studies were carried out to determine the optimum dose of ethanolic extract, DCM-F, and Aq-F which exhibited maximal hypoglycemic activity. Rats were randomly divided into ten groups (n=6) with normal control (received only water). Rats treated with the graded dose of EtOH-Et, DCM-F, and Aq-F (50, 100 and 200 mg/kg of b.wt.) in normal saline by intraperitonieal injection. The food was withdrawn during the 6th h of the experiment. After determination of blood glucose level at 6th h, the food was given to the animals and blood glucose was measured again at 24th h using One-Touch Select Simple (Johnson & Johnson Pvt. Ltd. Mumbai, India). From the data obtained, the optimum dosage was fixed as (100 mg/kg b.wt.) for further studies.
Preparation of diabetic animal models: T1DM induced in rats by the single injection of STZ (60 mg/kg b.wt.; I.P.) dissolved in 0.1 mol/L citrate buffer, pH 4.5. After 72 h, rats with >250 mg/ dl blood glucose confirmed diabetes (blood glucose level) was used in the study. T2DM induced using overnight fasting rats by a single-dose injection of STZ (60 mg/kg b.wt.; I.P.), 15 min after the rats injection of nicotinamide (I.P., 120 mg/kg b.wt.). T2DM ensured by the rats was given glucose tolerance test, and fasting insulin resistance index (FIRI) was compared control, and T2DM control rats. FIRI was calculated via blood sampling from ocular sinus and measurements of fasting insulin and glucose levels. T2DM confirmed rats undergone 12 h fasting, and then 40% glucose solution with the concentration of (2 g/kg of b.wt.; I.P.) was injected to rats. After 30, 60, and 120 min of after glucose injection the blood-glucose level was measured [25].
Administration of extract and fractions to the diabetic animal models: Overnight fasted diabetic rats were divided into eight groups of seven rats each. Extracts and fractions were orally administered for 14 days (daily once) are mentioned in Table 1.
| No. of group | Treatment |
|---|---|
| Group 1 | Normal rats+0.5 % CMC |
| Group 2 | T1DM control+0.5 % CMC |
| Group 3 | T2DM control+0.5 % CMC |
| Group 4 | T1DM rats+EtOH-Et (100 mg/kg b.wt.) suspended in 0.5% CMC |
| Group 5 | T1DM rats+DCM-F (100 mg/kg b.wt.) suspended in 0.5% CMC |
| Group 6 | T1DM rats+Aq-F (100 mg/kg b.wt.) suspended in 0.5% CMC |
| Group 7 | T2DM rats+EtOH-Et (100 mg/kg b.wt.) suspended in 0.5% CMC |
| Group 8 | T2DM rats+DCM-F (100 mg/kg b.wt.) suspended in 0.5% CMC |
| Group 9 | T2DM rats+Aq-F (100 mg/kg b.wt.) suspended in 0.5% CMC |
Table 1: Administration of extract and fractions to the diabetic animal models.
On the first and last day of the experiment, the body weight of rats was noted. On 0th, 7th, and 14th-day fasting blood glucose estimated by the glucose oxidase-peroxidase method [26]. According to the kit manufacturer's instruction serum cholesterol, triglycerides, and HDL-C were also evaluated in normal and diabetic rats.
Statistical analysis
The data was calculated in mean ± standard error mean. All the parameters were analyzed using One-way analysis of variance (ANOVA) followed by the Dunnet's Multiple Range Test (DMRT) using Graph Pad Instat 4.0.
Results
Phytochemical studies
Phytochemical studies indicated phenolics, saponin terpenoids, and flavonoids were present in the EtOH extract and the aqueous fraction of R. mucronata. DCM-F exhibited the presence of alkaloids in a high amount based on the sedimentation when compared to other phyto-constituents like carbohydrates, diterpenes, flavonoids, fats and oil, glycosides, phenols, proteins, resins, saponin and tannins (Table 2). Total alkaloids, flavonoids and phenols in the ethanolic extract, DCM-F and Aq-F were shown in Table 3.
| Phytochemicals | Tests | Interference | EtOH | DCM-F | Aq-F |
|---|---|---|---|---|---|
| Alkaloids | Mayer’s reagent | Formation of yellow cream (p) | +++ | ++++ | + |
| Wagner’s reagent | Formation of reddish brown (p) | ++ | +++ | ++ | |
| Carbohydrates | Molish’s reagent | Formation of violet ring | + | + | + |
| Benedict’s reagent | Formation of orange red (p) | + | + | + | |
| Fehling’s reagent | Formation of red (p) | + | + | + | |
| Saponin | Foam | Produce foam | - | - | - |
| Glycosides | Brontrager’s | Formation of rose pink colour | + | + | + |
| Fats and Oil | Filter paper press | Formation of oily stain | + | + | + |
| Resins | Acetone water | Appearance of turbidity | - | - | - |
| Phenols | Ferric chloride | Formation of bluish black | ++ | ++ | + |
| Tannins | Gelatin | Formation of white (p) | + | + | + |
| Diterpenes | Copper acetate | Formation of bright green colour | + | + | ++ |
| Flavonoids | Alkaline reagent | Formation of yellow colour | ++ | + | + |
| Lead acetate | Formation of yellow colour | + | + | + | |
| Shinoda | Formation of magenta colour | + | + | + | |
| Zinc HCl | Formation of magenta colour | + | + | + | |
| Proteins | Xanthoproteic | Formation of yellow colour | ++ | ++ | ++ |
| ++++: high content; +++: medium content; ++/+: low content; _: no content (content was evaluated as the sediment or the intensity of colour). | |||||
Table 2: Qualitative phytochemical screening of ethanolic extract, dichloromethane and aqueous fraction of R. mucronata.
| Extracts | Total alkaloids (mg/g) | Total Phenols (mg/g) | Total flavonoids (mg/g) |
|---|---|---|---|
| EtOH-Et | 29.34 ± 0.31 | 30.34 ± 0.74 | 11.34 ± 0.17 |
| DCM-F | 250.53 ± 0.51 | 190.53 ± 0.15 | 75.53 ± 0.14 |
| Aq-F | 120.42 ± 0.06 | 95.45 ± 0.05 | 12.42 ± 0.06 |
Table 3: Yield of different extracts and quantification of phytochemicals.
Effect of R. mucronata extracts and fractions in the changes of behavior and appearance
The impact of ethanolic extract, dichloromethane and an aqueous portion of R. mucronata on the physical and the behavioral changes of mice are appeared in Table 4. There were no mortality or any indications of poisonous quality observed in animals, up to seven days. The behavioral changes of animals were seen after the sixth hour (first day), third day and followed by seventh day after the administration of test samples treated animals were normal and did not show noteworthy changes in conduct, skin impacts, breathing, hindrance in nourishment admission and water utilization, postural irregularities and balding. There were no noteworthy changes in body weight. All the animals showed a normal growth in body weight without major difference between both control and treated animals. The toxicity study confirmed that LD50 value of EtOH, DCM-F, and Aq-F was more than 2000 mg/kg of animal body weight.
| Observation | Control group | Test group | ||||
|---|---|---|---|---|---|---|
| 1st day | 3rd day | 7th day | 1st day | 3rd day | 7th day | |
| Toxicity at 50 mg/kg | 0/3a | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Toxicity at 300 mg/kg | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 |
| Toxicity at 2000 mg/kg | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 1/3 |
| Body weight (g) | 22.7 ± 0.56 | 20.7 ± 0.65 | 21.7 ± 0.79 | 25.1 ± 0.85 | 25.7 ± 0.75 | 24.7 ± 0.25 |
| Skin and fur | Normal | Normal | Normal | Normal | Normal | Normal |
| Eyes | Normal | Normal | Normal | Normal | Normal | Normal |
| Mucous membrane | Normal | Normal | Normal | Normal | Normal | Normal |
| Behavioural patterns | Normal | Normal | Normal | Normal | Normal | Normal |
| Salivation | Normal | Normal | Normal | Normal | Normal | Normal |
| Lethargy | Normal | Normal | Normal | Normal | Normal | Normal |
| Sleep | Normal | Normal | Normal | Normal | Normal | Normal |
| Diarrhoea | No | No | No | No | No | No |
| Tremors | No | No | No | No | No | No |
| a =Number of dead mice/ number of mice used; values of body weight are mean ± SD (n=3). | ||||||
Table 4: Behavioural observations and appearance of control and test groups-65432.
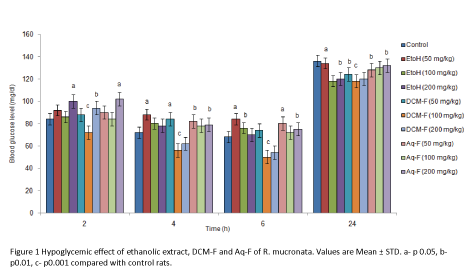
Hypoglycemic activity of EtOH extracts and its fractions of R. mucronata
Normoglycemic rats were used in order to evaluate the ability of the extract and fractions of R. mucronata in applying a hypoglycemic effect. The extract and fractions were seen to exhibit moderate hypoglycemic activity (Figure 1). 100 mg/ kg dose of the test samples exhibited the maximum glucoselowering potential at the time interval of 2 h following extract administration when compared with other higher doses. It is reducing the blood glucose level by 20% as compared with the control group. Overall, it was observed that the response elicited was not dose- dependent and that the dose of 100 mg/kg was the most effective, displaying a consistent blood glucose lowering effect on normoglycemic rats. Rats were fed after the 6th h reading and blood glucose again recorded at 24 h in the fed state. This reading showed that the 100 mg/kg b.wt. of R. mucronata exerted a prolonged effect on the blood glucose level since rats treated with the EtOH-Et, DCM-F and Aq-F were found to have significantly lower levels of blood glucose than the control. None of the doses studied lowered blood glucose level below 50 mg/ dl and, hence, it may be stated that the hypoglycemic effect was moderately mild.
Anti-hyperglycemic and anti-hyperlipidemic effect
The present study could complete with 52 rats, and 11 rats died during the execution of the study. Blood glucose level increased in the diabetic control group of both T1DM and T2DM. A significant reduction (P<0.01) in the blood glucose levels was brought about in diabetic rats by daily administration of the DCM-F than compared to the ethanolic extract, and aqueous basic fraction (Table 5). Treatment with DCM-F caused significant improvement in the HDL-C level as compared with the diabetic control groups. Serum cholesterol and triglyceride levels were decreased significantly (P<0.01) by DCM-F in T1DM and T2DM rats (Table 6).
| Group | 0th day | 7th day | 14th day |
|---|---|---|---|
| Normal | 102 ± 56 | 082 ± 48 | 125 ± 34 |
| T1DM C | 615 ± 32 | 945 ± 37 | 556 ± 13 |
| T2DM C | 783 ± 56 | 044 ± 42 | 753 ± 24 |
| T1DM+EE (100 mg/kg) | 643 ± 29 | 413 ± 66 | 564 ± 29a |
| T1DM+DCM-F (100 mg/kg) | 651 ± 13 | 399 ± 29 | 547± 19b |
| T1DM+Aq-F (100 mg/kg) | 662 ± 26 | 427 ±16 | 573 ± 28a |
| T2DM+EE (100 mg/kg) | 733 ± 31 | 543 ± 28 | 512± 30c |
| T2DM+DCM-F (100 mg/kg) | 881 ± 45 | 621±31 | 467 ± 39d |
| T2DM+Aq-F (100 mg/kg) | 842 ± 29 | 674 ± 27 | 493 ± 22c |
| Values are aP<0.05, bP<0.01 vs IDDM; cP<0.05, dP<0.01 vs NIDDM | |||
Table 5: Effect of ethanolic extract, dichloromethane, and acid aqueous fraction on blood glucose level in experimental rats.
| Group | TC | TG | HDL |
|---|---|---|---|
| Normal | 872 ± 36 | 824 ± 45 | 373 ± 26 |
| T1DM C | 547 ± 76 | 505 ± 51 | 282 ± 22 |
| T2DM C | 575 ± 54 | 564 ± 32 | 243 ± 25 |
| T1DM+EE (100 mg/kg) | 174 ± 37a | 042 ± 17a | 308± 33a |
| T1DM+DCM-F (100 mg/kg) | 554 ± 20b | 471± 41b | 201± 47b |
| T1DM+Aq-F (100 mg/kg) | 122 ± 39a | 973 ± 28a | 341 ± 28a |
| T2DM+EE (100 mg/kg) | 215 ± 1c | 051 ± 20c | 306 ± 18c |
| T2DM+DCM-F (100 mg/kg) | 166 ± 43d | 023 ± 19d | 335± 36d |
| T2DM+Aq-F (100 mg/kg) | 268 ± 29c | 065± 37c | 299± 31c |
| aP<0.05, bP<0.01 vs IDDM; cP<0.05, dP<0.01 vs NIDDM | |||
Table 6: Effect of ethanolic extract, dichloromethane and acid aqueous fraction on serum lipid profile in experimental rats on 21st day.
Discussion
The ethno-medicinal valuable of mangrove plants had an enormous amount of secondary metabolites when compared to terrestrial plants. The World health organization (WHO) suggested the utilization of plants for the management of diabetes mellitus and encouraged scientific evaluation of its anti-hyperglycemic properties [17,27,28]. In order to explore mangrove plants and maximize the therapeutic benefits, efforts should be geared toward novel anti-diabetic agent development. Therefore, we evaluated R. mucronata for possible management of diabetes mellitus. Dichloromethane fractions of different plant extract viz., Bridelia ndellensis, Ficus odorata, Rhizophora apiculata and Kalanchoe pinnata [29-31] support the anti-hyperglycemic effect DCM-F of R. mucronata in diabetic rats.
Toxicity studies in mice could be used to assess extracts of biological sources for different therapeutic application in a minimum dosage level [32]. It is difficult in definitively evaluating the safety of these test samples are established to have an adequate possible for development into drugs. However, the effects of the test samples on nervous and respiratory systems should be evaluated prior to human exposure because it is not defined in OECD-423 [33].
Based on the literature information, the oral route administration is the most convenient because of their slow absorption, cost effective and painless to the animals. Since the extract or fractions are administered orally, the animals should fast before taking the dose to avoid the effect of test samples disturbed by food and other factors. Although there is a trouble about animal experimental data to humans, because toxicity results of mice offer a superior prophecy for human LD50 contrast to rats for long-term studies [34]. Therefore, the present study was particularly designed to investigate the toxicity of ethanolic extract, dichloromethane and an aqueous fraction of R. mucronata by using acute oral toxicity analysis. The physical appearances such as eyes, fur and skin were revealed to be normal and slight increases in the body weight indicates that the test samples of R. mucronata have an insignificant level of cause on animal growth. The results of food intake and water consumption indicate there was no interruption of test samples of R. mucronata in metabolism of carbohydrate, fat and protein [35]. It is exciting to observe that the hyperglycemic state in OGTT down within 2 h after administration of DCM-F in an identical way to that of glibenclamide compared to ethanolic extract and aqueous fraction. Thus, it is rational to presume that DCM-F and glibenclamide might enhance the release of insulin or imitate insulin-like action.
STZ is diabetogenic agent destroys the insulin producing β-cells by inducing necrosis. Due to the structural resemblance of STZ is entering into the pancreatic cell through glucose transporter 2 in the plasma membrane [36]. STZ alone induces T1DM with the symptoms include severe glycemia, glucosuria, polyphagia, polydipsia and body weight loss, which occur chiefly because of loss of β-cells. In the present study, the hyperglycemic state and body weight loss in the experimental rats indicates STZ destroyed β-cells which results in the induction of insulindependent diabetes mellitus. Numerous studies performed to understand the pathogenesis of diabetes mellitus. However, complex pathology of human noninsulin dependent diabetes still unknown. Nicotinamide a water-soluble vitamin plays a valuable function in delaying the beginning of T1DM in nonobese animals [37]. Treatment of pre-diabetic with nicotinamide induces a diabetic with stable metabolic alterations and reduction in pancreatic insulin [38]. In the present study, we used STZ/nicotinamide diabetic rat model, with abnormal glucose tolerance and FIRI to verify T2DM in rats. Our results show that the ethanolic extract and dichloromethane fraction of R. mucronata demonstrated anti-hyperglycemic effects in T1DM rats. The inactivity of the extract may indicate that the extract also acts by stimulation of the pancreatic cells and it needs the action of β-cells. In the T2DM diabetic model rats, the ethanolic extract and dichloromethane fraction of R. mucronata showed an efficient anti-diabetic activity comparable to that of the aqueous fraction. Thus, indicates that the extract may act on β-cells like sulfonylurea drugs to stimulate insulin secretion. There are some similar results have been reported with A. occidentale aqueous leaf extract [39]. The dichloromethane and ethanolic extract of R. mucronata did not show any hypoglycemic effect in T2DM rats on fasting condition, it can be assumed that this extract may stimulate insulin secretion in a glucose-dependent manner. On the other hand, the hypoglycemic effect of the extract in rats indicates an inhibition of intestinal glucose absorption and the stimulation of the glucagon-like peptide (GLP-1) which is also a glucose-dependent insulin secretagogue [40].
3-hydroxy-3-methyl-glutaryl coenzyme A reductase (HMGCo-A reductase) is one of the important enzymes involved in cholesterol production. However, the insufficient secretion of insulin cannot inhibit the HMGCo-A reductase as a result, hyperlipidemic condition occurs in diabetes mellitus patients [41]. In the mean time, the uncontrolled diabetic circumstances in experimental rats directly proportional to increase in the total cholesterol, triglycerides and decreases in high-density lipoprotein [42]. In the present study, the T2DM rats treated with dichloromethane fraction of R. mucronata shows significantly decreased level of total cholesterol, triglycerides together with elevated level of high-density lipoprotein cholesterol. It clearly elucidates the anti-diabetic and anti-hyperlipidemic functional context of R. mucronata.
Conclusion
In this study, the DCM-F of the ethanol extract is evidenced for vital hypoglycemic and lipidemic effects in T2DM rats by the active metabolites of the R. mucronata in this fraction. However, the further purification and characterization of the metabolites are in progress to give imperative outcome for anti diabetic drug development from the R. mucronata.
Acknowledgements
The authors gratefully acknowledge to the Director, Centre for Advanced Study in Marine Biology, Faculty of Marine Science, Annamalai University, India for providing all support during the study period.
Conflict of Interest
All authors declared there is no conflict of interest.
References
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN (2009) Hyperglycemic crises in adult patients with diabetes. Diabetes care 32:1335-1343.
- Ganz ML, Wintfeld N, Li Q, Alas V, Langer J, et al. (2014) The association of body mass index with the risk of type 2 diabetes: a case–control study nested in an electronic health records system in the United States. Diabetol Metab Syndr 6: 50.
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, et al. (2012) Management of hyperglycemia in type 2 diabetes: A patient-centered approach. Diabetes care 35: 1364-1379.
- Kaliamurthi S, Selvaraj G, Thirugnanasambandam R (2014) Documentation of hypoglycemic and wound healing plants in Kodiyampalayam coastal village (southeast coast of India). J Coast Life Med 2:642-647.
- Gurudeeban S, Kaliamurthi S, Sheik HS, Thiruganasambandam R (2014) Molecular docking, isolation and biological evaluation of Rhizophora mucronata flavonoids as anti-nociceptive agents. Biomed Preventive Nutr 4:555-560.
- Manilal A, Merdekios B, Idhayadhulla A, Muthukumar C, Melkie M (2015) An in vitro antagonistic efficacy validation of Rhizophora mucronata. Asian Pac J Trop Dis 5:28-32.
- Pandey AK, Gupta PP, Lal VK (2014) Hypoglycemic effect of Rhizophora mucronatain streptozotocin induced diabetic rats. J Complement Integr Med 11:179-183.
- Ramanathan T, Hariharan B, Ganesan K (2008) Antidiabetic activity of a coastal mangrove leaves of Rhizophora mucronata. Plant Arch 8:931-933.
- Ravikumar S, Inbaneson SJ, Suganthi P, Gnanadesigan M (2011) In vitro antiplasmodial activity of ethanolic extracts of mangrove plants from South East coast of India against chloroquine-sensitive Plasmodium falciparum. Parasitol Res 108:873-878.
- Rege A, Chowdhary A (2014) Evaluation Of Alpha-Amylase And Alpha-Glucosidase Inhibitory Activities of Rhizophora mucronata. Int J Pharrm Sci Res 5:2261.
- Rege AA, Ambaye RY, Deshmukh RA (2012) Evaluation of in vitro inhibitory effect of selected plants and Shilajit on HIV-reverse transcriptase. Indian J Nat Prod Resour 3: 145-151.
- Hardoko ES, Puspitasari Y, Amalia R (2015) Study of ripe Rhizophora mucronata fruit flour as functional food for antidiabetic. Int Food Res J 22:953-959.
- Gurudeeban S, Ramanathan T, Satyavani K (2013) Antimicrobial and Radical Scavenging Effect of Alkaloid Elutes from Rhizophora mucronata. PharmChem J 47: 50-54.
- Rohini R, Das AK (2010) Triterpenoids from the stem bark of Rhizophora mucronata. Nat Prod Res 24:197-202.
- Arumugam S, Palanisamy D, Sambandam RT (2014) Identification of bioactive compounds of Rhizophora mucronata poir. leaves using supercritical fluid extraction and GC-MS.World J Pharm Pharmaceut Sci 3: 1621-1631.
- Satyavani K, Gurudeeban S, Manigandan V, Rajamanickam E, Ramanathan T (2015) Chemical Compositions of Medicinal Mangrove Species Acanthus ilicifolius, Excoecaria agallocha, Rhizophora apiculata and Rhizophora mucronata. Curr Res Chem 7:1-8.
- Gurudeeban S, Satyavani K, Ramanathan T, Ravikumar P (2012) Dipeptidyl peptidase IV inhibitors derived from a mangrove flora Rhizophora mucronata: An in silico approach. Bangladesh J Pharmacol 7: 203-210.
- Gurudeeban S, Satyavani K, Ramanathan T, Balasubramanian T (2012) An in silico approach of alpha-ketoglutarate dependent dioxygenase FTO inhibitors derived from Rhizophora mucronata. Drug Invent Today4: 594-598.
- Manigandan V, Gurudeeban S, Satyavani K, Ramanathan T (2014) Molecular Docking Studies of RhizophoramucronataAlkaloids Against NeuroinÃÆïÃâìÃâââ¬Å¡ammatory Marker Cyclooxygenase 2. Int J Biol Chem 8:91-99.
- Evans WC (1997) Trease and Evans Pharmacognosy. 14thedn. Harcourt Brace and Company. Asia Pvt. Ltd, Singapore, pp: 226-227.
- Ajanal M, Gundkalle MB, Nayak SU (2012) Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer. Ancient Sci Life 31: 198.
- Vabkova J, Neugebauerova J (2013) Determination of total phenolic content, total flavonoid content and frap in culinary herbs in relation to harvest time. Acta Univ Agric et Silvic Mendel Brun 60: 67-172.
- Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10: 178-182.
- Wilhelm KP, Maibach HI (2008) OECD guidelines for testing of chemicals. Dermatoxicology, 7th edn CRC Press, Boca Raton, pp:303-305.
- Duncan MH, Singh BM, Wise PH, Carter G, Alagh band-zadeh J (1995) A simple measure of insulin resistance. Lancet 346: 120-121.
- Trinder P (1969) Determination of glucose in blood using glucose oxidasewith an alternative oxygen acceptor. Ann Clin Biochem 6: 24-27.
- Rahman M, Siddika A, Bhadra B, Rahman S, Agarwala B, et al. (2010) Antihyperglycemic activity studies on methanol extractof Petrea volubilis L.(Verbenaceae) leaves and Excoecaria agallocha L.(Euphorbiaceae) stems. Adv Nat Appl Sci 4: 361-364.
- Nabeel MA, Kathiresan K, Manivannan S (2010) Antidiabetic activity of the mangrove species Ceriops decandra in alloxanÃÆâÃâââ¬ÃâÃÂinduced diabetic rats. J Diabetes 2: 97-103.
- Degollado JV, Yolo RT, Santiago LA (2014) Hypoglycemic effect and in vitro antioxidant activity of the dichloromethane fraction from the leaves of ficus odorata (blanco) merr.(moraceae). Int J Res Develop Pharma Life Sci 3: 1163-1173.
- Selvaraj G, Kaliamurthi S, Thirugnasambandan R (2016) Effect of Glycosin alkaloid from Rhizophora apiculata in non-insulin dependent diabetic rats and its mechanism of action: In vivo and in silico studies. Phytomedicine 23: 632-640.
- Patil SB, Dongare VR, Kulkarni CR, Joglekar MM, Arvindekar AU (2013) Antidiabetic activity of Kalanchoe pinnata in streptozotocin-induced diabetic rats by glucose independent insulin secretagogue action. Pharmaceut Biol 51:1411-1418.
- Ajani EO, Sabiu S, Bamisaye FA, Ibrahim S, Salau BA (2015) Evaluation of the acute and sub-acute toxicity effects of ethanolic leaves extract of Lagenaria brevifolia (Bitter gourd) on hepatic and renal function of rats. Eur J Med Plants 5: 210.
- Angelina M, Dewijanti ID, Hartati S, Meilawati L (2014) Acute oral toxicity and brine shrimp lethality of Pterocarpus indicus standardized ethanol extract. Int J Pharm Tech Res 6: 676-685.
- Syahmi ARM, Vijayarathna S, Sasidharan S, Yoga Latha L, Kwan YP, et al. (2010) Acute oral toxicity and brine shrimp lethality of Elaeis guineensis Jacq., (Oil Palm leaf) methanol extract. Molecules 15: 8111-8121.
- Vaghasiya YK, Shukla VJ, Chanda SV (2011) Acute Oral Toxicity Study of Pluchea arguta Boiss Extract in Mice. J Pharmacol Toxicol 6: 113-123.
- Nayak Y, Hillemane V, Daroji VK, Jayashree BS, Unnikrishnan MK (2014) Antidiabetic activity of benzopyrone analogues in nicotinamide-Streptozotocin induced Type 2 diabetes in rats. Sci World J 2014: 1-12.
- Azooz MM, Alzahrani AM, Youssef MM (2013) The potential role of seed priming with ascorbic acid and nicotinamide and their interactions to enhance salt tolerance in broad bean (Vicia faba L.). Aust J Crop Sci 7: 2091-2100.
- Szkudelska K, Nogowski L, Szkudelski T (2014) Adipocyte dysfunction in rats with streptozotocin-nicotinamideÃÆâÃâââ¬ÃâÃÂinduced diabetes. Int J Exp Pathol 95: 86-94.
- Sokeng DS, Kamtchouing P, Watcho P, Jatsa HB, Moundipa PF, et al. (2001) Hypoglycemic activity of Anacardium occidentale L. aqueous extract in normal and streptozotocin-induced diabetic rats. J Diabetes Res 36: 1-9.
- Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, et al. (1993) Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 268: 19650-19655.
- Betterridge J (2002) Lipid disorders in diabetes mellitus. In Textbook of Diabetes. Pickup J, Williams G. London, Blackwell Science, pp: 551-553.
- Sharma SB, Balomajumder C, Roy P (2008) Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem Toxicol 46:2376-2383.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences