In-Vitro Antioxidant Properties of Nauclea Latifolia Unriped Fruits
Martin Ganyam1*, Chur TN, Atsembe DM, Idikwu DA and Garba SE
Department of Biochemistry, Federal University of Agriculture Makurdi Benue State, Nigeria
- *Corresponding Author:
- Martin Ganyam
Department of Biochemistry, Federal University of Agriculture Makurdi Benue State, Nigeria
Tel: 2347035505243
E-mail: ganyamm@yahoo.com
Received Date:February 14, 2022, Manuscript No. IPJHM-22-12353; Editor assigned date: Februar y 17 2022, preQC No. IPJHM-22-12353 (PQ); Reviewed date: March 03, 2022, QC No. IPJHM-22-12353; Revised date: April 18, 2022, Manuscript No. IPJHM-22-12353 (R); Published date: April 26, 2022, DOI: 10.36648/2472-0151/22.8.001
Citation:Ganyam M, Chur TN, Atsembe DM, Idikwu DA, Garba SE (2022) In-Vitro Antioxidant Properties of Nauclea Latifolia Unriped Fruits. Herb Med Vol:8 No:4
Abstract
The study aimed to investigate the in-vitro antioxidant properties of methanol and N-hexane crude extracts of Nauclea latifolia unriped fruits. Qualitative and quantitative phytochemical analysis of methanol and N-hexane crude extracts of Nauclea latifolia unriped fruits were determined spectrophotometrically in duplicates and their antioxidant activities, examined in-vitro using 2,2-Di Phenyl-1-Picryl Hydrazyl (DPPH), hydrogen peroxide (H2O2) and Ferric Reducing Antioxidant Power assay (FRAP), all were done according to standard laboratory practices. Statistical analysis were carried out by one-way analysis of variance (ANOVA) supplemented by Duncan multiple range test using SPSS version 20. Results obtained revealed, the presence of phenol, flavonoids, tannins and alkaloids in both extracts, however, methanol extract revealed a higher concentration of tannin (0.994 ± 0.070 mg/ml) TAE when compared with N-hexane extract with a value of 0.263 ± 0.028 (mg/ml) TAE. Also, N-hexane extract revealed a higher percentage (15%) of alkaloid when compared to methanol extract (10%). The percentage inhibition of oxidation in both extracts decreased down the column with respect to decrease in concentration of the extracts. Methanol extracts exhibited higher percentage inhibition on DPPH (84.3%) at 100 mg/ml, N-hexane exhibited a higher inhibition of 88.8% for H2O2 at 100 mg/ml. Methanol and N-hexane extracts revealed a higher percentage inhibition of 87.6% and 88.0% respectively at 10 mg/ml for FRAP. The absorbance recorded also correlated with the level of percentage inhibitions of methanol and N-hexane crude extracts of Nauclea latifolia unripe fruits. The study thus revealed methanol and N-hexane extracts of unriped fruits of Nauclea latifolia possesses in-vitro antioxidant properties at the highest concentrations tested, and may be used in management of oxidative stress related diseases.
Keywords
Nauclea latifolia; 2,2-di Phenyl-1-Picryl Hydrazyl (DPPH); Hydrogen peroxide (H2O2); Ferric Reducing Antioxidant Power assay (FRAP)
Introduction
Oxidative stress is defined as a disturbance in the equilibrium between Free Radicals (FR), Reactive Oxygen Species (ROS) and the endogenous defense mechanisms (McCord, 2000). Free radicals are produced as part of normal cellular function. They may also be produced by endogenous and environmental sources. Endogenous sources include mitochondrial leak, respiratory burst, enzyme reactions and auto-oxidant reactions. Environmental sources include cigarette smoke, pollutants (such as ozone and nitrogen dioxide), ultraviolet light, ionizing radiation, and xenobiotics. The most important free radicals produced are the oxygen derivatives such as the superoxide radical (O2•¯), and the hydroxyl free radical (OH•) [1-4]. Other examples of free radicals include nitric oxide (NO•), nitrogen dioxide (NO2•), peroxyl (ROO•), alkoxyl radicals (RO•) and lipid peroxyl (LOO•). These are highly reactive species, capable of damaging biologically relevant macromolecules such as Deoxyribo Nucleic Acid (DNA), proteins, carbohydrates and lipids in the nucleus and membranes of cells.
The human body has several mechanisms to counteract oxidative stress by producing antioxidants, which are either naturally produced in situ, or externally supplied through foods and/or supplements. Endogenous and exogenous antioxidants act as ‘‘free radical scavengers”” by preventing and repairing damages caused by Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) thereby enhancing the immune defense and lowering the risk of cancer and other degenerative disease [5-7]. Drug formulations that are antioxidant based are used in the prevention and treatment of complex diseases which include atherosclerosis, stroke, diabetes, Alzheimer’s disease, and cancer [8]. However, other natural and alternative plant sources known for antioxidant activities also exist.
Nauclea latifolia Smith (family: Rubiaceae) is a straggling, evergreen, multistemmed shrub or small tree native to tropical Africa and Asia. It normally produces interesting flowers, edible, but not appealing, large red ball fruits with long projecting stamens [9-11]. Commonly used parts of Nauclea latifolia include the leaves, roots, stem, and fruits.
Phytochemical screening of the ethanolic extract of the fruits revealed the presence of alkaloids, tannins, saponins, phytates, flavonoids and cyanogenic glycosides, while proximate analysis revealed that the fruits are rich in proteins, fiber, carbohydrates, moisture, dry matter, vitamins (A, B1, B2, C, and E). The fruits were also shown to contain essential minerals such as copper, iron, cobalt, calcium, magnesium, zinc and phosphorus [12]. Isolation and characterization of some of the active constituents of the ripe and unripe fruits of the plants led to isolation of some phthalates and fatty acid esters [13-15]. Several other studies have confirmed the health potentials of Nauclea latifolia. Some of its medicinal effects include antimalarial, antidiabeti, antihypertensive, antipyretic and antinociceptive, and anti-inflammatory and analgesic activities [16-20]. It is also known to have a strong antibacterial property [21]. This study is therefore aimed to investigate the antioxidant properties of methanolic and N-hexane crudes extracts of unripe fruits of Nauclea latifolia.
Materials and methods
Study design
50 g each of the powdered sample were accurately weighed using a Digital scale (Techne 7) and were macerated in 300 ml (0.3 L) of each of the methanol (99.9%) and N-hexane (85%) respectively for 48 hrs with intermittent shaking. The mixture was filtered using whatman's filter paper No 1 to obtain a solution devoid of solids, for methanol and N- hexane respectively. The crude extracts were further concentrated in a water bath set at 60°C for 72 hrs [22-25].
Subjects
Healthy and fresh unriped fruits of Nauclea latifolia were collected from Yandev, Gboko Local Government Area of Benue State, in the month of February, 2021 and were identified and authenticated in the Department of Botany, University of Agriculture Makurdi. The sliced unriped fruits were shade dried at room temperature for two weeks before being pulverized into smooth powder with an electric grinder. The resulting powder was stored in a polythene bag pending extraction.
Determination of percentage yield of methanolic and N-hexane Extracts of Nauclea latifolia
Percentage yield of the extract were calculated as follows.
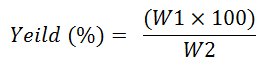
Where,
W1=Weight of extract after evaporation of solvent.
W2=Dry weight of the sample before extraction.
Preliminary Phytochemical Screening
Test for Phenol
A few quantity of the sample (0.5 g) was dissolved in water and filtered; to this 2 ml of the 10 % aqueous sodium hydroxide was later added to produce a yellow colouration. A change in colour from yellow to colourless on addition of dilute hydrochloric acid was an indication for the presence of phenols [26-28].
Test for Flavonoid
A few quantity of the sample (0.5 g) was dissolved in water and filtered. To this 2 ml of the filtrate, few drops 10% ferric chloride solution was added to produce a green-blue or violet colouration. A change in colour from green-blue or violet on addition of dilute ferric chloride was an indication of the presence of flavonoids [29-32].
Test for Tannin
Sample (0.5 g) was stirred with 10 ml of distilled water and then filtered. Few drops of 1% ferric chloride solution were added to 2 ml of the filtrate. The occurrence of a blue-black, green or blue-green precipitate indicated the presence of tannins [33].
Test for Alkaloid
A few quantity of the sample (0.5 g) was stirred with 5 ml of 1% aqueous HCl on water bath and then filtered. Of the filtrate, 1 ml was taken individually into 2 test tubes. To the first portion, few drops of Dragendorff's reagent were added; occurrence of orange-red precipitate was taken as positive. To the second 1 ml, Mayer's reagent was added and appearance of buff-coloured precipitate was an indication for the presence of alkaloids [34-36].
Quantitative Phytochemical Screening of Methanolic and N-hexane Crude Extract of Nauclea latifolia
Total phenolic content
The total phenolic content was evaluated by adopting a modified spectrophotometric method described by Singleton and Rossi with slight modifications. 1 g of both methanolic and N-hexane extracts were dissolved in 10 ml methanol and N-hexane respectively [37-40].
0.5 ml of the filtrate (methanol and N-hexane) were added into separate test tube, followed by 0.5 ml of 1:10 dilution of Folin Ciocalteu reagent. After few minutes, 0.5 ml of 15% Na2CO3 solution was added and the solution was made up to 4 ml with distil water. The reaction mixture was allowed to stand for 20 minutes. The absorbance of the mixture was read at 760 nm and tannic acid was used as standard. Phenolics concentration was calculated using the formula, Absorbance of sample/Absorbance of standard × concentration of standard, where the concentration of standard=0.1 mg/ml [41].
Total flavonoid content
This was determined spectrophotometrically using the method described by Jia with some modifications. 1 g of both methanolic and N- hexane extracts were dissolved in 10 ml methanol and N-hexane respectively [42-45].
1 ml of the filtrate (methanol and N-hexane) was measured into separate test tube, and 75 µL of 5% NaNO3 solution was added to each test tube. After five minutes, 150 µL of 10% Al2Cl3 was added followed by 500 µL of 10% NaOH and 275 µL of H2O (distill water) after six minutes. The mixture was read using the ultra violet spectrometer at 510 nm and quercetin was used as standard. Flavonoid concentration was calculated using the formula, Absorbance of sample/Absorbance of standard×concentration of standard, where the concentration of standard=0.1 mg/ml [46].
Total tannin content
Quantity of tannins was determined by using the spectrophotometer method [47-50]. 1 g of both methanolic and N-hexane extracts were dissolved in 10 ml methanol and N-hexane respectively.
1 ml of the filtered sample (methanol and N-hexane) were pipetted out into separate test tubes, and mixed with 800 µL of 0.1 M FeCl3 in 0.1 M HCl and 800 µL K4Fe (CN) 6.3H2O. The absorbance was measured with a spectrophotometer at 395 nm wavelength within 10 minutes. Tannic acid was used as standard. Tannin concentration was calculated using the formula, Absorbance of sample/Absorbance of standard × concentration of standard, where the concentration of standard=0.1 mg/ml. concentration was calculated using the formula, Absorbance of sample/Absorbance of standard×concentration of standard, where the concentration of standard=0.1 mg/ml.
Total alkaloid content
The total alkaloid content was evaluated by adopting the method described by Adeyemi [51-60].
1 g of both methanolic and N-hexane extracts were dissolved in 10 ml methanol and N-hexane respectively. 2 ml of the filtered sample (methanol and N-hexane) were pipetted out into separate beaker/conical flask and were titrated with concentrated ammonial until precipitation was completed. The whole solution was allowed to settle down for five minutes [61-64].
Two filter papers were weighed and samples were filtered. The residues obtained were collected and washed with concentrated NH3. The filter papers were labeled and allow to dry. The residues were alkaloid, which were then dried and weighed. The differences in masses were recorded [65].
The percentage of total alkaloids was calculated using equation
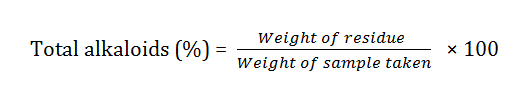
Antioxidant activities of methanolic and N-hexane crude extracts of Nauclea latifolia Scavenging effect on 2, 2-Di Phenyl-1-Picryl Hydrazyl radical (DPPH)
The ability to scavenge the stable free radical DPPH or antioxidant activity was determined using the DPPH free radical scavenging method [66-69]. 0.21 g of 2, 2-Di Phenyl-1-Picry Hydrazyl radical (DPPH), a stable radical was dissolved in methanol (100 ml). 2 g of each extracts were dissolved separately in 20 ml 99.9% methanol and 85% N-hexane respectively. To 3.0 ml of the methanolic solutions of DPPH was added 0.5 ml (500 µl) of each of the extracts with concentration 100 mg/ml. The same procedure was repeated for the extracts concentrations (50 mg/ml and 25 mg/ml) obtained from two fold serial dilution. The mixture was incubated in the dark for 10 minutes upon which the decrease in absorption at 517 nm of DPPH was measured [70]. The actual decrease in absorption was measured against that of the control and the percentage inhibition was also calculated. The same experiment was carried out on Butylated Hydroxyl Anisole (BHA), α-tocopherol and ascorbic acid which are known standards antioxidant [71]. All test and analysis were run in duplicates and the results obtained were averaged [72-75]. The Radical Scavenging Activity (RSA) was calculated as the percentage inhibition of DPPH discoloration using the equation below:
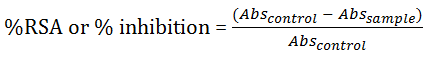
Where Abssample is the absorbance of the sample solution, Abscontrol is the absorbance of control. Absorbance of DPPH=2.115 [76].
Scavenging effect on Hydrogen Peroxide (H2O2)
The antioxidant activities of methanolic and N-hexane crude extract of Nauclea latifolia scavenging effect on hydrogen peroxide were determined according to Oloyede and Farombi [77-78]. A solution of 2 mM hydrogen peroxide was prepared in Phosphate Buffered-Saline (PBS) pH 7.4.2 g of each extracts were dissolved separately in 20 ml 99.9% methanol and 85% N-hexane respectively. To 3.0 ml of the H2O2 solution was added 0.5 ml of each of the extracts with concentration 100 mg/ml. The same procedure was repeated for the extracts concentrations (50 mg/ml and 25 mg/ml) obtained from two fold serial dilution. Decrease in absorbance of H2O2 at 285 nm was determined spectrophotometrically 10 minutes later against a blank solution containing the reagents in distil water without the test sample. All tests were run in duplicates and averaged. The same experiment was carried out on Butylated Hydroxy Anisole (BHA), ascorbic acid and α-tocopherol which are known antioxidant standards. Absorbance of H2O2=1.030 [79-82].
Antioxidant activity by ferric thiocyanate method
The antioxidant activities of methanolic and N-hexane extracts of Nauclea latifolia were determined by ferric thiocynate method [83-89]. 2 g of each extracts were dissolved separately in 20 ml 99.9% methanol and 85% N-hexane respectively. A mixture of 2 ml of sample in 2 ml 99.5% ethanol, 4 ml phosphate buffer (pH 7.0) and 2 ml of water was placed in a vial with a screw cap and placed in an oven at 600C in the dark for 10 minutes. To 0.1 ml (100 µl) of this sample solution with concentration 10 mg/ml, 10 ml of 75% ethanol and 0.1 ml of 30% ammonium thiocynate was added. After the addition of 0.1 ml of 2 × 10-2 M ferrous chloride in 3% hydrochloric acid to the reaction mixture, the absorbance of the red colour which developed was measured in 3 minutes at 500 nm. This procedure was repeated for 5 mg/ml and 2.5 mg/ml of extracts concentrations obtained from two fold serial dilutions. The control and standards were subjected to the same procedures as the sample, except that for the control, only solvent was added, and for the standard, sample was replaced with the same amount of Butylated Hydroxyl Anisole (BHA), ascorbic acid and α-tocopherol (reference compounds) [90-93]. The inhibition of lipid peroxidation in percentage was calculated using this equation: %Inhibition=1-(A1/A2) X 100
Where A1 was the absorbance of the test sample and A2 was the absorbance of control reaction. Absorbance of FRAP=2.766 [94].
Data presentation and statistical analyses
Results were expressed as mean ± Standard Deviation (SD) of replicate determinations. Statistical analyses of mean values were carried out by one-way analysis of variance (ANOVA) supplemented by Duncan multiple range test using SPSS version 20. Values were considered statistically significant at p ≤ 0.05 [95-100].
Results
Determination of percentage yield of methanolic and n-hexane extracts of unripe fruits of Nauclea latifolia
The determination of percentage yield of methanolic and N-hexane extracts of unripe fruits of Nauclea latifolia revealed that, N-hexane extracts had a higher yield (33.22%) as compare to that of methanolic extracts (28.78%) (Table 1) [101-105].
| Extracts | W2 (g) | W1 (g) | % Yield |
|---|---|---|---|
| Methanol | 50 | 14.39 | 28.78 |
| N-hexane | 50 | 16.61 | 33.22 |
| W1=weight of extract after evaporation of solvent.W=Dry weight of the sample before extraction.%=percentage yield. | |||
Table 1: Percentage yield of methanolic and N-haxane extracts of Nauclea latifolia.
Preliminary phytochemical screening
The preliminary phytochemical analysis of methanolic and N-hexane extracts of Nauclea latifolia unripe fruits as illustrated in (Table 2). Revealed the presence of all the phytochemicals that were screened for, which include; phenols, flavonoids, tannins and alkaloids [106-108]. Tannins were found to be present in very high concentration (+++) in the methanolic extracts of Nauclea latifolia unripe fruits, while the N-hexane extracts contained minimal concentration (+) of tannin and phenols [109].
| Phytochemicals | Methanolic extracts | N-hexane extracts |
|---|---|---|
| Phenols | + | + |
| Flavonoids | ++ | ++ |
| Tannins | +++ | + |
| Alkaloids | + | ++ |
| Key: +=minimally present++=moderately present+++=highly present | ||
Table 2: Preliminary phytochemical screening of methanolic and N-hexane crude extracts of Nauclea latifolia.
Phenols and alkaloids contained minimal concentration (+) in the methanolic extracts of N. latifolia unripe fruits. Flavonoids contained moderate concentration for both extracts [110].
Quantitative phytochemical screening of methanolic and N-hexane crude extracts of nauclea latifolia unripe fruits
The quantitative phytochemical composition of methanolic and N-hexane extracts of Nauclea latifolia unripe fruits revealed that the phytochemicals were in different concentration [111].
Tannins were the most abundant (0.994 ± 0.070) in the methanolic extracts, while having a lower concentration (0.263 ± 0.028) in the N-hexane extracts.
Flavonoids concentrations were high in both extracts (0.659 ± 0.001, 0.649 ± 0.110) for methanolic and N-hexane extracts respectively.
Phenol concentrations in both extracts were almost the same, having the least concentration (0.230 ± 0.014, 0.226 ± 0.002) for methanolic and N-hexane extracts respectively. Alkaloids were expressed in percentage with a moderate concentration (15%) in N-hexane extracts and a lower concentration (10%) in methanolic extracts (Table 3 and Table 4).
| Phytochemicals | Methanolic extracts | N-hexane extracts |
|---|---|---|
| Phenols (mg/ml)TAE | 0.230 + 0.014 | 0.226 + 0.002 |
| Flavonoids (mg/ml) QE | 0.659 + 0.001 | 0.649 + 0.110 |
| Tannins (mg/ml) TAE | 0.994 + 0.070 | 0.263 + 0.028 |
| Alkaloids (%) | 10 | 15 |
| Results are expressed as means + S.D; n=2.Key: QE=quercetin equivalence.TAE=Tannic acid equivalence.S.D=standard deviation. | ||
Table 3: Quantitative phytochemical screening of methanolic and N-hexane crude extracts of Nauclea latifolia unripe fruits.
| Concentration of extracts (mg/ml) | Methanolic extracts (%) | N-hexane extracts (%) | Alpha-tocopherol (%) | Ascorbic | BHA |
|---|---|---|---|---|---|
| Acid (%) | (%) | ||||
| 100 | 84.3 | 52.4 | 97.7 | 90 | 97.9 |
| 50 | 84.1 | 61.5 | 97.4 | 75.3 | 91.6 |
| 25 | 67.4 | 68.9 | 53.6 | 40.6 | 89.1 |
Table 4: Percentage inhibition of Nauclea latifolia extracts (methanolic and N-hexane), alpha-tocopherol,ascorbic acid and BHA on 2,2-diphenyl-1-picryl hydrazyl (DPPH).
Percentage inhibition of methanolic and n-hexane crude extracts of Nauclea latifolia unripe fruits
Percentage inhibition of methanolic extracts, N-hexane extracts and standards on DPPH
The percentage inhibition of methanol and N-hexane extracts of unripe fruits of Nauclea latifolia along with antioxidant standards on 2,2-DiPhenyl-1-Picryl Hydrazyl (DPPH) revealed, methanolic extract with higher inhibition when compared to N-hexane extract. The inhibition decreases down the column with respect to decrease in the concentration of extracts (84.3%, 100 mg/ml; 84.1%, 50 mg/ml and 67.4, 25 mg/ml), except for the N-hexane extracts as the inhibition tends to increase with respect to decrease in concentration of extracts (52.4%, 100 mg/ml; 61.5%, 50 mg/ml and 68.9%, 25 mg/ml) (Table 5) [112].
| Concentration of extracts (mg/ml) | Methanolic extracts (%) | N-hexane extracts (%) | Alpha-tocopherol (%) | Ascorbic | BHA |
|---|---|---|---|---|---|
| Acid (%) | (%) | ||||
| 100 | 65.8 | 88.6 | 82.3 | 87.6 | 85.4 |
| 50 | 55.1 | 75 | 81.6 | 75.9 | 76 |
| 25 | 52.7 | 60.5 | 36.4 | 75.7 | 32.2 |
Table 5: Percentage inhibition of Nauclea latifolia extracts (methanolic and N-hexane), alpha-tocopherol, ascorbic acid and BHA on hydrogen peroxide (H2O2).
Percentage inhibition of methanolic extracts, N-hexane extracts and standards on Hydrogen peroxide (H2O2)
The percentage inhibition for both extracts and antioxidant standards were found decreasing down the column in a concentration-dependent manner.
N-hexane extracts had higher inhibitory action on H2O2 (88.6%, 100 mg/ml; 75.0, 50 mg/ml and 60.5, 25 mg/ml) when compared with the corresponding methanolic extracts and standards.
When the inhibition of methanolic extracts were compared with that of the standards, result revealed that, standards provides higher inhibitory action on H2O2 than the corresponding methanolics extracts (Table 6) [113-115].
| Concentration of extracts (mg/ml) | Methanolic extracts (%) | N-hexane extracts (%) | Alpha-tocopherol (%) | Ascorbic | BHA |
|---|---|---|---|---|---|
| Acid (%) | (%) | ||||
| 10 | 87.6 | 88 | 51.4 | 96.9 | 63.8 |
| 5 | 85.6 | 68.9 | 48.6 | 69.9 | 61 |
| 2.5 | 83 | 64.9 | 46.6 | 48 | 58 |
Table 6: Percentage inhibition of Nauclea latifolia extracts (methanolic and N-hexane), alpha-tocopherol, ascorbic acid and BHA on Ferric Reducing Antioxidant Power assay (FRAP).
Percentage inhibition of methanolic extracts, N-hexane extracts and standards on Ferric Reducing Antioxidant Power assay (FRAP)
Methanolic extracts of Nauclea latifolia unripe fruits had excellent inhibitory action (87.6%, 100 mg/ml; 85.6%, 50 mg/ml and 83.0%, 25 mg/ml) on FRAP than N-hexane extracts and the corresponding standards as illustrated in (Table 7). The percentage inhibition of extracts (methanol and N-hexane) and standards decreases down the column with respect to decrease in concentration of extracts and standards.
| Concentration of extracts (mg/ml) | Methanolic extracts | N-hexane extracts | Alpha-tocopherol | Ascorbic acid | BHA |
|---|---|---|---|---|---|
| 100 | 0.332 ± 0.001a | 1.006 ± 0.003c | 0.048 ± 0.016a | 0.212 ± 0.011a | 0.044 ± 0.008a |
| 50 | 0.336 ± 0.004a | 0.815 ± 0.026b | 0.055 ± 0.068a | 0.522 ± 0.001b | 0.177 ± 0.005b |
| 25 | 0.690 ± 0.014b | 0.658 ± 0.016a | 0.982 ± 0.141b | 1.256 ± 0.000c | 0.231 ± 0.043b |
| Values are expressed as mean ± S.D; n=2.Values with different superscript down the column are considered statistically significant p ≤ 0.05 | |||||
Table 7: Absorbance of the Scavenging effect of Nauclea latifolia extracts (methanolic and N-hexane) and standards on 2,2-Di Phenyl-1-Picryl Hydrazyl (DPPH).
Scavenging effect of Nauclea latifolia extracts (methanol and n-hexane)
Scavenging effect of Nauclea latifolia extracts (methanolic and n-hexane) on 2,2-diphenyl-1-picrylhydrazyl (DPPH)
The absorbance measurement of methanolic extracts, n-hexane extracts, α-tocopherol, ascorbic acid and BHA as illustrated by Table 8 revealed, methanolic extracts had strong scavenging activity on DPPH (0.332 ± 0.001, 0.336 ± 0.004 and 0.690 ± 0.014) than the extracts of N-hexane and standards. There was no significant (p ≤ 0.05) increase when the absorbance at 100 mg/ml was compared with that of 50 mg/ml. While the absorbance of methanolic extracts and standards decreases down the column with decreased concentration, the absorbance of N-hexane extracts tends to increase with decreased concentration. Values were considered statistically significant at p ≤ 0.05 [116].
Scavenging effect of Nauclea latifolia extracts (methanolic and n-hexane) on Hydrogen peroxide (H2O2)
The absorbance measurement of methanolic extracts, n-hexane extracts, α-tocopherol, ascorbic acid and BHA showed that, N-hexane extracts had strong scavenging activity on H2O2 (0.117 ± 0.002, 0.258 ± 0.035 and 0.407 ± 0.006) than the extracts of methanol, alha-tocopherol and BHA. No significant (p ≤ 0.05) difference was observed when absorbance at 100 mg/ml was compared with 50 mg/ml concentration, but when compared with the 25 mg/ml concentration, there was a significant (p ≤ 0.05) decrease. Ascorbic acid possessed the strongest scavenging activity on H2O2 (0.128 ± 0.016, 0.248 ± 0.014 and 0.250 ± 0.048) (Table 8) [117].
| Concentration of extracts (mg/ml) | Methanolic extracts | N-hexaneExtracts | Alpha-tocopherol | Ascorbic acid | BHA |
|---|---|---|---|---|---|
| 100 | 0.352 ± 0.001a | 0.117 ± 0.002a | 0.182 ± 0.033a | 0.128 ± 0.016a | 0.150 ± 0.071a |
| 50 | 0.462 ± 0.002b | 0.258 ± 0.035b | 2.251 ± 0.054c | 0.248 ± 0.014b | 2.307 ± 0.037c |
| 25 | 0.487 ± 0.008c | 0.407 ± 0.006c | 0.655 ± 0.001b | 0.250 ± 0.048b | 1.727 ± 0.004b |
| Values are expressed as mean ± S.D; n=2.Values with different superscript down the column are considered statistically significant p ≤ 0.05 | |||||
Table 8: Absorbance of the Scavenging effect of Nauclea latifolia extracts (methanolic and N-hexane) and standards on hydrogen peroxide (H2O2).
Scavenging effect of Nauclea latifolia extracts (methanol and n-hexane) on Ferric Reducing Antioxidant Power assay (FRAP)
The absorbance measurement of methanolic extracts, n-hexane extracts, and standards revealed, methanolic extracts scavenging activity on FRAP (0.352 ± 0.001, 0.398 ± 0.034 and 0.471 ± 0.004) was significantly higher (p ≤ 0.05) than that of N-hexane extracts and standards. (Table 9) [118].
| Concentration of extracts (mg/ml) | Methanolic extracts | N-hexaneExtracts | Alpha-tocopherol | Ascorbic acid | BHA |
|---|---|---|---|---|---|
| 10 | 0.342 ± 0.014a | 0.332 ± 0.001a | 1.343 ± 0.009a | 0.085± 0.010a | 1.000 ± 0.105a |
| 5 | 0.398 ± 0.034a | 0.860 ± 0.037ab | 1.422 ± 0.001ab | 0.833 ± 0.970a | 1.080 ± 0.014a |
| 2.5 | 0.471 ± 0.004b | 0.971 ± 0.305c | 1.477 ± 0.071b | 1.439 ± 0.001a | 1.163 ± 0.035a |
| Values are expressed as mean ± S.D; n=2.Values with different superscript down the column are considered statistically significant p ≤ 0.05 | |||||
Table 9: Absorbance of the Scavenging effect of Nauclea latifolia extracts (methanolic and N-hexane) and standards on Ferric Reducing Antioxidant Power Assay (FRAP).
Discussion
Essential source of new chemical substances with potential therapeutic effects is thought to be obtained from medicinal plants [119]. The antioxidant contents of medicinal plants may contribute to protection against diseases [120]. Natural antioxidants have attracted a great deal of public and scientific attention because of their health promoting effects [121]. An imbalance between the production of reactive oxygen species (ROS) and the activity of the antioxidant defences leads to oxidative stress [122]. In the pathology of several human diseases such as atherosclerosis, inflammation, cancer, rheumatoid arthritis, and neurodegenerative diseases like Alzheimer’s disease and multiple sclerosis, ROS have been implicated. Attention is on antioxidant agents of natural origin due to their abilities to scavenge free radicals [123]. Antioxidant capacity is associated with compounds that can protect a biological system against the damaging effect of ROS and Reactive Nitrogen Species (RNS) [124].
The presence of phenols, flavonoids, alkaloids and tannins in the unriped fruits of Nauclea latifolia methanolic and N-hexane extracts is an indication that these secondary plant metabolites have a synergistic effect on the various pharmacological properties reported by Onyesom [125].
Quantitative phytochemical analysis revealed, total flavonoid content were higher in both methanol (0.659 ± 0.001) and N-hexane (0.649 ± 0.110) extracts. Total phenol and tannin contents were significantly higher (p ≤ 0.05) in methanol extracts than the N-hexane extracts.
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical is used as a stable free radical to determine the antioxidant activity of natural compounds and the scavenging of stable radical (DPPH) is considered a valid and easy assay to evaluate scavenging activity of antioxidants [126]. In this assay, the deep violet colour of DPPH is reduced to α,α-diphenyl-β-picrylhydrazine (yellow coloured) when neutralised. The extent of the change in colour is proportional to the concentration and strength of the antioxidants. The results generated from this study demonstrated that the unriped fruits of Nauclea latifolia methanolic and N-hexane extracts possessed good free radical scavenging activity. This potential may be attributed to the appreciable amounts of phenolic and flavonoid content of both extracts. According to Kumar and Pandey [127]. Functional hydroxyl groups in flavonoids mediate their antioxidant effects by scavenging free radicals and/or by chelating metal ions. The B ring hydroxyl configuration is the most significant determinant of scavenging of ROS and RNS because it donates hydrogen and an electron to hydroxyl, peroxyl, and peroxynitrite radicals, stabilizing them and giving rise to a relatively stable flavonoids radical [128]. The structural requirement considered to be essential for effective radical scavenging by flavonoids is the presence of a 3', 4'-dihydroxy, i.e., a o-dihydroxy group (3',4'-catechol structure) in the B ring, possessing electron donating properties and being a radical target. In general, the radical-scavenging activity of flavonoids depends on the molecular structure and the substitution pattern of hydroxyl groups, i.e., on the availability of phenolic hydrogens and on the possibility of stabilization of the resulting phenoxyl radicals via hydrogen bonding or by expanded electron delocalization [129].
Mechanisms of antioxidant action can include (1) suppression of ROS formation either by inhibition of enzymes or by chelating trace elements involved in free radical generation; (2) scavenging ROS; and (3) up regulation or protection of antioxidant defenses [130]. Flavonoid action involves most of the mechanisms mentioned above. Some of the effects mediated by them may be the combined result of radical scavenging activity and the interaction with enzyme functions. Flavonoids inhibit the enzymes involved in ROS generation, that is, microsomal monooxygenase, glutathione S-transferase, mitochondrial succinoxidase, NADH oxidase, and so forth [131]. This has translated to the high percentage inhibition obtained as well.
N-hexane extracts possessed high scavenging activity on H2O2 than methanolic extracts. This is because; H2O2 partially oxidizes N-hexane due to chemical inertness of alkanes. When the absorbance of the three concentrations were compared, absorbance decreased significantly (p ≤ 0.05), in a concentration-dependent manner [132].
Donating electron to reduce ferric ion to ferrous ion is an indication of reducing power. The reducing capacity of a compound may be directly translated as an indicator of antioxidant activity [133]. The strong reducing power of Nauclea latifolia methanolic and N-hexane extracts might be as a result of its phenolic content, as they are good electron donors [134]. Both extracts possessed antioxidant activity on FRAP. However, it has been observed that the methanolic extracts exhibited strong activity with the increase in polarity, indicating that highly polar organic compounds may play important role in the antioxidant activities [135-136]. The antioxidant activity exhibited by extracts was greater than the reference compounds alpha tocoherol, however, the antioxidant activity possessed by ascorbic acid surpassed that of extracts and other standards (Alpha tocoherol and BHA).
Conclusion
This study provides evidence that Nauclea latifolia unriped fruits possessed significant antioxidant activities in a concentration dependent manner on (DPPH, H2O2, FRAP) which can be attributed to the phenolics, flavonoid, tannin, alkaloid and other bioactive compounds present in the extracts.
References
- Young IS, Woodside JV (2001) Antioxidants in health and disease. J Clin Pathol 54:176 -186
- Chatterjee M, Saluja R, Kanneganti S, Chinta, ikshit (2007) Biochemical and molecular evaluation of neutrophil NOS in spontaneously hypertensive rats. Cell Mol Biol 53:84-93
- Khalaf NA, Shakya AK, Al-Othman A, El-Agbar Z, Farah H, et al. (2008) “Antioxidant activity of some common plants. Turk J Biol 32:51–55
- James O, Ugbede HH (2011) “Hypocholesterolemic effects of Nauclea latifolia (Smith) fruit studied in albino rats.” Am J Trop Med Pub Hea 1:11–21
- Agyare C, Mensah AY, Osei-Asante S (2006) Antimicrobial activity and phytochemical studies of some medicinal plants from Ghana. Bol Latinoam Caribe Plantas Med Aromat 5:113-117
- Eze SO, Obinwa E (2014) Phytochemical and nutrient evaluation of the leaves and fruits of N. latifolia (Uvuru-ilu). Comm Appl Sci 2:8-24
- Fadipe AL (2014) Isolation and characterization of di- (1-hexen5-yl) phthalate and monoethyl phthalate from the ethyl acetate extract of unripe fruits of Nauclea latifolia. Int j boil pharm allied sci 3:776-784
- Benoit-Vical FA, Valentin V, Cournac Y, P´elissier M, Malli´e, et al. (1998) “In-vitro antiplasmodial activity of stem and root extracts of Nauclea latifolia S.M. (Rubiaceae).” J Ethnopharmacol 61: 173–178
[Crossref] [Google scholar] [Indexed]
- Gidado A, Ameh DA, Atawodi SE (2005) “Effect of Nauclea latifolia leaves aqueous extracts on blood glucose levels of normal and alloxan-induced diabetic rats.” Afr J Biotechnol 4:91–93
[Crossref] [Google scholar] [Indexed]
- Nworgu ZAM, Onwukaeme DN, Afolayan AJ, Amaechina FC, Ayinde BA (2008) “Preliminary Studies of blood lowering effects of Nauclea latifolia in rats.” Afr J Pharmacy Pharmacol 2:37–41
- Taiwe GS, Bum EN, Talla E, Dimo T, Weiss N, et al. (2011) “Antipyretic and antinociceptive effects of Nauclea latifolia root decoction and possible mechanisms of action". Pharm Biol 49:15–2 5
- Goji ADT, Mohammed A, Tanko Y, Ezekiel I, Okpanchi AO, et al. (2010) “A study of the antiinflammatory and analgesic activities of aqueous extract of Nauclea latifolia leaves in Rodent.” Asian J Med Sci 2:244–247
- Okiei W, Ogunlesi M, Osibote EA, Binutu MK, Ademoye MA, et al. (2011) “Comparative studies of the antimicrobial activity of components of different polarities from the leaves of Nauclea latifolia.” Res J MedPlant 5:321–329
[Crossref]
- Trease GE, Evans WC (2002) Phenols and Phenolic Glycosides, In: Trease and Evans Pharmacognosy and Biliere. Tindall, London.
- Sofowora A (1993) Medicinal Plants and Traditional Medicine in Africa. (2ndedition). Spectrum Books Limited, Ibadan. 191-289
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic 16:144-158 [Crossref]
[Googlescholar][Indexed]
- Jia ZS, Tang MC, Wu JM (1999) The determination of flavonoid contents in muberry and their scavenging effects on superoxide radicals. Food Chem 64:555-599
[Crossref] [Google scholar] [Indexed]
- Adeyemi TOA, Ogboru RO, Idowu OD, Owoeye EA, Isese MO (2014) Phytochemical screening and health potentials of Morinda lucida Benth. Int J Innov Sci Res 11:515-519
[Indexed]
- Hatano T, Kagawa H, Yasuhora T, Okuta T (1988) Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem Pharm Bull 36:200-209
[Crossref] [Google scholar] [Indexed]
- Gulcin IM, Oktay OK, Aslan A (2002) Determination of antioxidant activity of Linchen Cetraria islandica (L). J Ethnopharmacol 79:325-329
[Crossref] [Google scholar] [Indexed]
- Mutee AF, Salhimi SM, Yam MF, Lim CP, Abdullah GZ, et al. (2010) In-vivo anti-inflammatory and in-vitro antioxidant activities of Peperomia pellucida. Int J Pharmacol 6:686-690
- Oloyede GK, and Farombi OE (2010) Antioxidant Properties Crinum ornatum Bulb Extract. World J Chem 1:32-36
- Mackie RC, McCartney (1989) In Practical Medicinal Microbiology (3rd edtion), Churchill Livingstone (Publishers), London, 100-491
- Eisner T (1990) “Chemical prospecting: a call for action,” in Ecology, Economic and Ethics: The Broken Circles, F.E. Bormanand S.R. Kellert, Editions. Yale University Press.
- Saeed N, Khan MR, Shabbir M (2012) “Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L,” BMC Complement Altern Med 12:221
- Anwar F, Jamil A, Iqbal S, Sheikh MA (2006) “Antioxidant activity of various plant extracts under ambient and accelerated storage of sunflower oil.” Grasas y Aceites 57:189-197
[Crossref] [Google scholar] [Indexed]
- krov´ankov´a S, Mi?surcov´a L, Mach°u L (2012) “Antioxidant activity and protecting health effects of common medicinal plants,” Adv Food Nutr Res 67:75-139
[Crossref] [Googlescholar] [Indexed]
- Osawa T, Kavakishi S, Namiki M, Kuroda Y, Shankal DM, et al. (1990) Antimutagenesis and Anticarcinogenesis Mechanisms II. Plenum, New York, NY, USA.
- Karadag A, Ozcelik B, Saner S (2009) “Review of methods to determine antioxidant capacities.” Food Anal Methods 2:41–60
- Onyesom I, Osioma E, Okereke PC (2015) Nauclea latifolia aqueous leaf extract eliminates hepatic and cerebral Plasmodium berghei parasite in experimental mice. Asian Pac J Trop Biomed 5:546–551
- Suhaj M (2006) “Spice antioxidants isolation and their antiradical activity: a review,” J Food Compost Anal 19:531–537
[Crossref][Googlescholar][Indexed]
- Ozturk M, Aydogmus Ozturk F, Duru ME, Topcu G (2007) “Antioxidant activity of stem and root extracts of Rhubarb (Rheumribes): an edible medicinal plant,” Food Chem 103:623–630
- Maizura M, Aminah A, Wan Aida WM (2011) “Total phenolic content and antioxidant activity of kesum, ginger (Zingiber officinale) and turmeric (Curcuma longa)extract.” Int Food Res J 18:529–534
- Kumar S, Pandey AK (2013) “Phenolic content, reducing power and membrane protective activities of Solanum xanthocarpum root extracts,” Vegetos 26:301–307
- Cao G, Sofic E, Prior RL (1997) “Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships.” Free Radic Biol Med 22:749–760
[Crossref] [Google scholar] [Indexed]
- Rice-Evans A, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933-956
[Crossref] [Googlescholar] [Indexed]
- Halliwell B, Gutteridge JM (2015) Free Radicals in Biology and Medicine. Oxford University Press, New York.
- Mishra A, Kumar S, Pandey AK (2013) “Scientific validation of the medicinal efficacy of Tinospora cordifolia,” Scientific World J 2013:292934
- Brown JE, Khodr H, Hider RC, Rice-Evans C (1998) “Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties.” Biochem J 330:1173–1178
[Crossref] [Google scholar] [Indexed]
- Jean EG, Serge, K (1998) Catalyst: Kinetics and mechanistic studies. Can J Chem Eng 76:833-852
- Navghare VV, Dhawale SC (2017) Alexandria Journal Medicine 53:237–243
- Pereira A, Maraschin M (2015) Banana (Musa spp) from peel to pulp: Ethnopharmacology, source of bioactive compounds and its relevance for human health. J Ethnopharmacol 160:149–163
- Ganiyat KO, Sunday FA, Charles CN (2014) Antioxiant and toxicity screening of extracts obtained from Cyperus esculentus. Academia Arena.
- Abbiw DK (1990) Useful plants of Ghana. Intermediate Technology Publications and the Royal Botanic Gardens Kew, London.
- Abdullahi M, Amupitan JO, Oyawale AO, Okogun JI, Ibrahim K (2007) An ethnobotanical survey of indigenous flora for treating tuberculosis and other respiratory diseases in Niger State, Nigeria. J Phytomedicine Ther 12:1-12
[Crossref] [Google scholar] [Indexed]
- Adebowale EA (1993) Some ethno veterinary and traditional management practices in livestock production. In proceeding of a workshop on indigenous knowledge in agriculture and development, Ibadan, Nigeria 24-26
- Adefegha S, Oboh G (2011) Water Extractable phytochemicals from some Nigeria spices inhibit Fe2+ - Induced Lipid Peroxidation in Rat‘s Brain. Food Process Technol 1:2-6
- Ademola IO, Fagbemi BO, Idowu SO (2007) Antihelminthic efficacy of Nauclea latifolia extract against gastrointestinal nematodes of sheep in-vitro and in-vivo studies. Afr J Tradit Complement Altern Med 4:148-156
[Crossref] [Google scholar] [Indexed]
- Adjanohoun JE, Aboubakar N, Dramane K, Ebot ME, Ekpere JA, et al. (1996) Traditional medicine and pharmacopoeia: contribution to ethnobotanical and floristic studies in Cameroon. Addis-Ababa: OAU/STRC, Africa.
- Agoha RC (1981) "Medicinal plants of Nigeria" of retdikker jifaultat waskunden, natpurwe N enschopp, pen, Netherland. Public Health Nutr 6:251–256
- Alugoju P, Dinesh BJ, Latha P (2015) Free radicals: Properties, sources, targets and their implication in various diseases. Indian J Clin Biochem 30:11-26
[Crossref] [Google scholar] [Indexed]
- Arbonnier M (2000) Arbres, Arbustes et Lianes des Zones Seches d’Afrique de I’Ouest. Ist CIRAD Publishers, Paris.
- Arise RO, Akintola AA, Olarinoye JB, Balogun EA (2012) Effects of Nauclea latifolia stem on lipid profile and some Enzymes of Rat Liver and Kidney. Int J Pharmacol 10:23-39
[Crossref] [Google scholar] [Indexed]
- Arrigoni O, De Tullio MC (2002) Ascorbic acid: Much more than just an antioxidant. Biochimica et Biophysica Acta 1569:1-9
[Crossref] [Google scholar] [Indexed]
- Balogun ME, Besong EE, Obu DC, Obu MSU, Djobissie SFA (2016) Nauclea latifolia: A Medicinal, Economic and Pharmacological Review J Plant Res 6:34-52
- Berg JM, Tymoczko JL, Stryer L (2002) Biochemistry. (5th edition), Freeman WH and Co, New York.
- Bindhumol V, Chitra KC, Mathur PP (2003) A induces reactive oxygen species generation in the liver of male rats. Toxicology 188:117-124
[Crossref] [Google scholar] [Indexed]
- Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM (2002) Mitochondria: Regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med 33:755–764
[Crossref] [Google scholar] [Indexed]
- Burton GW, Joyce A, Ingold KU (1983) Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochemical and Biophysical Research Communication 221:281–290
[Crossref] [Google scholar] [Indexed]
- Butler J, Hoey BM (1993) The one-electron reduction potential of several substrates can be related to their reduction rates by cytochrome-P-450 reductase. Biochim Biophys Acta 1161:73–78
[Crossref] [Google scholar] [Indexed]
- Cheung CC, Zheng GJ, Li AM, Richardson BJ, Lam PK (2001) Relationship between tissue concentrations of polycyclic aromatic hydrocarbons and antioxidative responses of marine mussels. Aquat Toxicol 52:189-203
[Crossref] [Google scholar] [Indexed]
- Coe FC, Anderson GJ (1996) "Screening of Medicinal plants for bioactive compounds”. J Ethnopharmacol 3:429–431
- Deeni Y, Hussain H (1991) Screening for antimicrobial activity and for alkaloids of Nauclea latifolia. J Ethnopharmacol 35:91–96
[Crossref] [Google scholar] [Indexed]
- Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, et al. (2004) “Free radicals and antioxidants in human health: current status and future prospects.” J Assoc Physicians India 52:794–804
- Duarte TL, Lunec J (2005) When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res 39:671–686
[Crossref] [Google scholar] [Indexed]
- Elujoba AA (1995) Female infertility in the hands of traditional birth attendants in South-West Nigeria. Fitoterapia 66:239–248
- Esimore CO, Ebebe IM, Chah KF (2003) Comparative antibacterial effect of Psidium guajava aqueous extract. J Trop Med Plants 4:185-189
- Etukudoh I (2013) Ethnobotany: Conventional and Traditional uses of plants. Verdict Press, Uyo 116 -117
- Fahey RC (2001) Novel thiols of prokaryotes. Annu Rev Microbiol 55:333-356
[Crossref] [Google scholar] [Indexed]
- Fairlamb AH, Cerami A (1992) Metabolism and functions of trypanothione in the Kinetoplastida. Annu Rev Microbiol 46:695729
[Crossref] [Google scholar] [Indexed]
- Fern K (2008) Calopogonium caerulleum. Useful Tropical Plants Database 2014/feedipedia.
- Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
[Crossref] [Google scholar] [Indexed]
- Gill LS (1992) Ethnomedical Uses of Plants in Nigeria. Uniben Press. 276.
- Giovannucci E (1998) "Plant Bioactive Components: Phytochemistry”. J Biol Res 33:159–165
- Hacisevki A (2009) An overview of ascorbic acid biochemistry. J Fac Pharm 38:233-255
[Crossref] [Google scholar] [Indexed]
- Hayes D, McLellan LI (1999) Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31:273-300
[Crossref] [Google scholar] [Indexed]
- Hayes J, Flanagan J, Jowsey I (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
[Crossref] [Google scholar] [Indexed]
- Hayes JD, Pulford DJ (1995) The glutathione-S-transferase supergene family: regulation of resistance. Crit Rev Biochem Mol Biol 30:445–600
[Crossref] [Google scholar] [Indexed]
- Hayes JD, Pickett CB, Mantle B (1990) Glutathione-S-transferases and drug resistance. Cancer Cells 2:15-22
- Herbert VD (1994) "The antioxidant supplements myth." Am J Clin Nutr 5:66-69
- Hiner AN, Raven EL, Thorneley RN, Garcia-Canovas F, Rodriguez-Lopez JN (2002) Mechanisms of compound I formation in heme peroxidases. J Inorg Biochem 91:27–34
[Crossref] [
- Ho YS, Xiong Y, Ma W, Spector A, Ho DS (2004) Mice lacking catalase develop normally but show differential sensitivity to oxidant tissue injury. J Biol Chem 279:32804–32812
[Crossref] [Google scholar] [Indexed]
- Howard AC, Anna K, McNeil AK, McNeil PL (2011) Promotion of plasma membrane repair by vitamin E. Nat Commun 20:597
[Crossref] [Google scholar] [Indexed]
- Igboko DO (1983) Phytochemical studies on Garcinia Kola, Heckel M.Sc Thesis, University of Nigeria, Nsukka 202
- Imlay JA (2003) Pathways of oxidative damage. Annu Rev Microbiol 57:395–418
[Crossref] [Google scholar] [Indexed]
- Isah Y, Ndukwe IG, Joseph OA (2012) Isolation and bioactivity of pentacyclic triterpenoid (Betunilic acid) from the bark of Sarcocephalus latifolius (Smith Bruce). Journal Natural Sciences Research 2:13-23
- Iwu MM (1993) Handbook of African Medicinal Plants. (2nd Edition), CRC Press, Florida.
- Justesen U, Knuthsen P (2011) "Composition of flavonoids in fresh herbs" Food Chem 73:245-250
[Crossref] [Google scholar] [Indexed]
- Karou SD, Tchacondo T, lboudo DP, Simpore J (2011) SubSaharan Rubiaciaea. A review of their traditional uses, phytochemistry and biological activities. Pak J Biol Sci 14:149-169
[Crossref] [Google scholar] [Indexed]
- Kerharo J (1974) Historic and Ethnopharmacognosic Review on the Belief and Traditional Practices in the Treatment of Sleeping Sickness in West Africa. Bull Soc Med Afr Noire Lang Fr 19:400-410
- Kovacic P, Pozos RS, Somanathan R, Shangari N, OBrien PJ (2005) Mechanism of mitochondrial uncouplers, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure–activity relationships. Curr Med Chem 12:2601–2623
- Kumar S, Mishra A, Pandey AK (2013) “Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in-vitro models,” BMC Complement Altern Med 13:120
- Lamidi ME, Oliver R, Faurel L, Debrauwer L, Nze E, et al. (1995) Quinovic acid glycosides from Nauclea diderichic. Planta Med 61:280 – 281
- Linster CL, Van Schaftingen E (2007) Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J 274:1–22
- Lotito SB, Free B (2011) Consumption of flavonoid rich food and increased plasma anti-oxidant capacity in humans. Free Radic Biol Med 41:1727-1746
- Maduiyi I (1983) Biochemical and Pharmacological Studies of active principles of the seeds of Garcina Kola Heckel M.Sc Thesis, University of Nigeria, Nsukka 108
- Maitera ON, Khan ME,James TF (2011) Phytochemical analysis and the chemotherapeutics of leaves and stembark of Nauclea latifolia grown in Hong, Adamawa State Nigeria. Asian JPlant Sci 1:16-22
- Mannervik B, Danielson UH (1988) Glutathione transferases-structure and catalytic activity. CRC Crit Rev Biochem 23:283–337
- McCordJM(2000)The evolution of free radicals and oxidative stress. Am J Med 108: 652-659
- Meister A, Anderson M (1983) "Glutathione". Annu Rev Biochem 52: 711-760
- Michel A (2004) Trees, Shrubs and Lianas of West African latifolia stems bark against ovine nematodes. Fitoterapia 72: 12-21
- Mosialou E, Ekström G, Adang AE, Morgenstern R (1993) Evidence that rat liver microsomal glutathione transferase is responsible for glutathione dependent protection against lipid peroxidation. Biochem Pharmacol 45:1645–1651
- Antioxidants in health and disease
- Mustacich D, Powis G (2000) Thioredoxin reductase. Biochem J 346:1–8
[Crossref] [Googlescholar] [Indexed]
- Nordberg J, Arner ES (2001) Reactive oxygen species, antioxidants, andthe mammalian thioredoxin system. Free Radic Biol Med 31:1287–1312
- Nozik-Grayck E, Suliman H, Piantadosi C (2005) Extracellular superoxide dismutase. Int J Biochem Cell Biol 37:2466–2471
- Obeagu EI (2018) A review on free radicals and antioxidant. Infect Disord Drug Targets 4:123-133
- Okigbo BN (1995) Neglected plants of Agriculture and Nutritional Importance of Traditional Farming Systems in Tropical Africa. Acta Horticulture 5:13–15
- Okwori AEJ, Okeke CI, Uzoechina A, Etukudoh NS, Amali MN, et al. (2008) The antibacterial potentials of Nauclea Afrjbiotechnol 7:1394-1399
- Okwu DE (2001) Improving the Nutrition Value of Cassava tapioca meal withlocal Nutrac Funct Med Foods 3:43–51
- Okwu DE (2001) "Evaluation of the Chemical Composition of indigenous Spices and Flavouring agents.” Glob J Pure Appl 8:455–459
- Orwa C, Mutua A, Kindt R, Jamnadass R (2009) Agroforestree database: a tree reference and selection guide version 4.
- Oye GL (1990) Studies on antimalarial action of cryptolepis sanguinolenta extract. Proceeding of International Symposium on East-West medicine, Seoul, Korea.
- Pedro A, Antonio P (1998) A new Indole Alkaloid from Sarcocephalus Latifolius. Heterocycles 48:885– 891 [Crossref]
- Pickett CB, Lu AY (1989) Glutathione-S-transferases:gene structure, regulation, and biological function. Annu Rev Biochem 58:743-764
- Pigeolet E, Corbisier P, Houbion A, Lambert D, Michiels C, et al. (1990) Glutathione peroxidase, superoxide dismutase and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51:283–297
- Prohaska JR (1991) Changes in Cu, Zn-superoxide dismutase, cytochrome C oxidase, glutathiohne peroxidase and glutathione transferase activities in copper-deficient mice and rats. J Nutr 121:355–363
- Reitman S, Frankel S (1957) A colourimetric method for determination of Serum glutamate-oxaloacete and pyruvate transaminases. Am J Clin Pathol 28:56-63
- Ross IA (2005) Chemical Constituents, Traditional and Modern Medical Uses". Medicinal Plants of the World. Humana Totowa, NJ, England.
- Rouhier N, Lemaire SD, Jacquot JP (2008) The role of glutathione in photosynthetic organisms: Emerging functions for glutaredoxins and glutathionylation. Annu Rev Plant Biol 59:143-166
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi CY, et al. (2004) Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 6:289–300
- Singh RL, Sharma S, Singh P (2014) Antioxidants: Their Health Benefits and Plant Sources, Phytochemicals of nutraceutical importance. CABI Publishing, India.
- Starkov AA (2008) The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 1147:37–52
- Tappel ME, Chaudiere J, Tappel AL (1982) Glutathione peroxidase activities of animaltissues. Comp Biochem Physiol B 73:945–949
- Tran K, Wong JT, Lee E, Chan AC, Choy PC, et al. (1996) Vitamin E potentiates arachidonate release and phospholipase A2 activity in rat heart myoblastic cells. Biochem J 319:385–391
- Ulusu NN, Tandogan B (2007) Purification and kinetic properties of glutathione reductase from bovine liver. Mol Cell Biochem 303:45–51
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J, et al. (2004) Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem 266:37–56
[Crossref] [Googlescholar] [Indexed]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. The International Int J Biochem Cell Biol 39:44–84
- Vasileva B (1969) Plants Medicinales de Guinea Conakry, Republic de Guinea.
- Verma RS, Mhta A, Srivastava N (2007) In-vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pestic Biochem Physiol 88:191-196
- Williams RJ, Spencer JP, Rice TC (2012) Flavonoids antioxidant molecules. Free Radic Biol Med 36:838 – 849
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND, et al. (2004) Glutathione Metabolism and Its Implications for Health. J Nutr 134:489-492
- Yang H, Shi MJ, Van Remmen H, Chen X, Vijg J, et al. (2003) Reduction of pressor response to vasoconstrictor agents by overexpression of catalase in mice. Am J Hypertens 16:1–5
- Young HT, Petterson VJ (1995) Characterization of Food Flavonoid components in Kiwi Fruits. J Sci Food Agric 36:352–358
- Zelko IN, Mariani TJ, Folz RJ (2002) Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and ECSOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 33:337–349

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
