Comparative Study of Different Ayurvedic Loha Bhasam: Physico-Chemical and Structural Characterization
Gaurav Goswami*, Mona Semalty and Ajay Semalty
Published Date: 2025-01-10Gaurav Goswami*, Mona Semalty and Ajay Semalty
Department of Pharmaceutical Science, Hemvati Nandan Bahuguna Garhwal University, Srinagar Garhwal, 246174, Uttarakhand, India
- *Corresponding Author:
- Gaurav Goswami Department of Pharmaceutical Science, Hemvati Nandan Bahuguna Garhwal University, Srinagar Garhwal, 246174, Uttarakhand, India E-mail: gauravgoswami33@outlook.com
Received Date: June 18, 2023, Manuscript No. IPJHM-23-16985; Editor assigned date: June 21, 2023, PreQC No. IPJHM-23-16985 (PQ); Reviewed date: July 06, 2023, QC No. IPJHM-23-16985; Revised date: January 03, 2025, Manuscript No. IPJHM-23-16985 (R); Published date: January 10, 2025, DOI: 10.36648/2472-0151.11.1.002
Citation: Goswami G, Semalty M, Semalty A (2025) Comparative Study of Different Ayurvedic Loha Bhasam: Physico-Chemical and Structural Characterization. Herb Med Vol:11 No:2
Abstract
Background: Ayurveda's precious gift to humanity is the ancient medical practice known as Bhasma. Bhasma, which means "ash" in Ayurvedic, is a well-known effective remedy. These particular metal base preparations have a long history on the Indian peninsula and require extensive scientific validation through physico chemical research in order to be accepted by the contemporary scientific community. To understand the crystal profiles, particle size, chemical component and trace elements analysis of the pharmaceuticals, many methods including loss on drying, ash value, acid insoluble ash, total ash value, pH and sophisticated instrumental analysis like XRD, FTIR, SEM, etc. were explored.
Results: Five samples' XRD analyses revealed that they have somewhat amorphous structural characteristics. In five endothermic peaks in the range of 1 to 89 degrees Celsius were visible in the graph plot for all five samples. Therefore, an effort has been undertaken to compile a database of five such medications using contemporary analytical techniques. It may be said that while there weren't many comparative differences in the batch, the comparison based outcomes were superior. Bhasmas, also known as herbo metallic remedies, are concocted from metallic and herbal elements. One of the iron based herbo metallic preparations used in Ayurvedic medicine to treat a variety of conditions brought on by a lack of iron is called Lauha Bhasma (LB). Normal purification (samanya sodhana), special purification (vishesha sodhana), drying in the sun (bhanupaka), heating in a pan (sthalipaka) and then calcination (putapaka) using Triphala kwatha as a medium at a temperature of 650Ë?C in an Electric Muffle Furnace (EMF) and maintained for an hour are the steps involved in the preparation of LB. LB is submitted to several physicochemical analyses and cutting edge analytical techniques, including Scanning Electron Microscopy (SEM), Fourier Transform Infrared Spectroscopy (FTIR) and X-ray Spectroscopy (XRD). Ayurvedic loha Bhasam differs from market samples in various ways. The results indicate that these processes were required to achieve a decent quality of bhasma and also to make it acceptable during the Bhasmikarna process, according to the organoleptic characteristics, preliminary test, and physicochemical result of LB from data from physicochemical analysis, it was discovered that LB was made in 21 puta, with an average crystalline size of 57.23 nm as determined by XRD spectra. SEM analysis demonstrates the LB's fine grained, uniform sized particle structure. Fe is included in the EDX graph as a prominent element (75.43%). Different organic moieties that increase the therapeutic activity and cause bhasma to manifest strongly are suggested by FTIR spectra. 'Lauha' (Iron) Bhasma is largely utilised in Ayurveda to treat human ailments caused by an iron shortage.
Keywords
Ash value; Acid insoluble value; Limit tests for heavy metals; XRD; FTIR; Loha Bhasam
Introduction
The ancient healing science known as Ayurveda was created approximately 3000 B.C. Its name, which is a mix of the Sanskrit words Ayush (life) and Veda (science or knowledge), means "science of life" or "life knowledge". Modern biomedicine began to politically, intellectually and economically dominate Ayurveda in the twentieth century, giving rise to modern Ayurveda and global Ayurveda. Geographically positioned on the Indian subcontinent, modern Ayurveda has a tendency to become more secular by downplaying its magical and mythical elements [1]. The holistic Ayurvedic approach uses a number of blood issues in the management and treatment of illnesses. In Ayurveda, loha bhasam is utilised for drugs with metal components. Ayurvedic medications come in a variety of forms. After proper Sodhana (an Ayurvedic purifying method), impure metal. The Bhasmikarana (calcinations) process, which transforms metal into its specifically desired chemical component known as Bhasmas, is a very well-liked Ayurvedic medical procedure. It provides considerable medical advantages as well as eliminating the metal's toxicity. To demonstrate the qualitative effectiveness of the manufactured Bhasma, some classic qualitative tests are used in Ayurvedic philosophy [2]. However, the composition and structure of the Bhasma cannot be determined quantitatively by using these conventional methods. Validation is crucial if you want any drug to be accepted by the international scientific community for the application of an alternating external magnetic field or photo electron production using X-rays for the noninvasive localised target killing of cancer cells, especially in sensitive areas of the human body as the brain, spinal cord and lungs [3].
Loha Bhasam are byproducts of metal based ash and they differ in their therapeutic effects and method of classification. All ash comes from commercially available base. They help treat anaemia among other conditions. Clinical testing was conducted on relatively few samples. One of the causes of loha bhasma's low acceptance. Characterising the loha Bhasma on an elemental and structural level and contrasting it with The physico chemical evaluation of our pharmaceuticals of interest included qualitative analysis such as loss on drying, total ash, water soluble extractive, etc. Namburi Phased Spot Test (NST) performed chromatographic qualitative analysis. X-Ray Diffraction (XRD) was utilised to determine the type of chemical, whether it was crystalline or amorphous and the size of the crystals found in loha Bhasma. Since loha Bhasma is heated during processing, it is unlikely that any organic molecules will be present. The loha Bhasma contains a variety of components, including FTIR for chemical compounds and SEM for morphology. The goal of health care is to treat and prevent disease by harmonising the body, mind and spirit. Through food, herbal medicines, yoga and meditation, exercise, a healthy lifestyle and body cleaning, this 5000 year old practise aims to bring the body into harmony with nature. According to ayurveda, three doshas-vata (for transportation), pitta (for metabolism) and kapha (for storage) are in charge of maintaining the body's natural balance. The body's biological systems operate normally when the three doshas are in harmony and any distortion, disturbance, or imbalance between these doshas may result in a sick condition. The natural flow of "prana" (life force energy) is disrupted when dosha falls out of balance, which results in a buildup of poisonous waste in the body, mind and spirit that leads to sickness. According to the Ayurvedic philosophy, the energies of the five fundamental elements ether, air, fire, water and earth interact to create the entire cosmos. The combinations of the five elements known as vata (air and ether), pitta (fire and water) and kapha (water and earth) appear as patterns across all of creation. In contrast, biomedicine is based on a reductionist view of health and illness and prioritises the eradication of pathology. The most common kind of a chronic, excruciatingly painful condition that affects several joints and results in swelling and devastating deformity in most people is rheumatoid arthritis. Localised application of plant extracts, such as Semecarpus anacardium or marking nut, which cause chemical cauterization and surface burn like reactions, provides pain relief for aching, swollen joints. Similar cauterization can be accomplished by using hot Agnikarma probes made of iron, copper, or gold. Rasashastra is the earliest text to mention Bhasma. The materials known as "Rasa dravyaas" are the focus of this specialised section of Ayurveda. Rasashastra's branch includes steps like Sodhana, Marana and Puta. A branch of Nepalese and Indian medicine known as Rasashastra deals primarily with metals, minerals, products of animal origin, toxic plants and their use in therapies. Benzodiazepines utilised in Rasashastra.
Bhasam: Ash is composed of minuscule particles. As a result, they can deliver pharmacological efficacy to the human body quickly and effectively. Particle size reduction makes it easier for the Bhasma to be absorbed and assimilated by the system. According to recent investigations, the herbal mineral Ayurvedic compositions that make up Bhasma have a huge benefit in maintaining a healthy bodily and mental state. Ayurveda, which has been practised on the Indian subcontinent since the 7th century A.D. and is highly regarded for treating a wide range of chronic illnesses, is recognised for its special metallic/mineral preparation called as Bhasma. It is also treated using herbal juice or decoction. A Sanskrit term with the meaning "ash" is "Bhasma." It derives from the roots "bha" and "sma," which both imply "ever." Ash is considered to be sacred in Hindu mythology and yoga. Bhasma is a form of medicinal force created through the calcination of stones, jewels, minerals, or metals in the traditional Ayurvedic medical system of the Himalayan region. Many different Bhasmas are available and are used to cure a variety of diseases. Bhasma is a term for the ash that results from cremation after the initial material has been put through a laborious purification process. This is followed by a reaction phase that involves the addition of additional minerals and herbal extracts. Bhasmas are the field of nanomedicine, which demonstrates the value of nanotechnology in the fields of healthcare, disease diagnosis, treatment and disease prevention. Since it is involved in so many metabolic processes, such as the transfer of oxygen, the production of deoxyribonucleic acid and the movement of electrons, Iron (Fe) is a crucial element for practically all living things. Lauha Bhasma is the name for the Ayurvedic incinerated Fe preparations. It is a herbo-metallic substance with a variety of medicinal uses [4]. To match nanotechnology and be compatible with it. Bhasma are also utilised as preventative medications in addition to being used to treat a number of ailments. Bhasma are categorised according to their look and colour. According to scientific classification, they are divided into categories according to the prominent metals and minerals they contain, such as Rajata Bhasma (silver), Tamra Bhasma (copper), Loha Bhasma (iron), Pravala Bhasma (shells), etc. (Table 1).
| Serial no. | Types | Used in | Metals |
|---|---|---|---|
| 1 | Tamra | Used in adjust tonicity, maintain body circulation | Cu, S, Hg |
| 2 | Abraka | Used in hepatic dysfunctions, leukemia, cystis fibrosis | Ca, K ,Si |
| 3 | Loha/lauh | In anemia, deficiency of iron | Fe2O3, Fe3O4 |
| 4 | Hiraka | Contraction on vein and blood clotting, heart pain | O, Na, K, Mg, |
| 5 | Naga | Diabetes, diarrhea, spleen and skin disorder | Pb, Arsenic disulfide |
| 6 | Trivanga | Diabetes | Pb, Zn, Sn |
Table 1: Types of Bhasma.
Materials and Methods
Characterization using physic-chemical and structural
Physico-chemical characteristics of samples A to E were assessed and their chemical composition was characterised using accepted techniques.
LOD or loss on drying: The Loss on Drying Test (LOD) determines how much water or volatile substances are present in a sample. Each air dried sample, which contains 1 gram of medication, was taken in 5 distinct petri dishes and weighed precisely at about 5 gm. After that, it spent an hour in an electronic oven at 110°C. In a desiccator, the item is allowed to cool to ambient temperature after being removed from the over and then it is weighted to the constant weigh.
Alcohol soluble extractive: In a closed flask for 24 hours, 5 gm of air dried samples from all 5 samples (each containing 1 g) were combined with 50 ml of alcohol (each drug containing 10 ml) at the prescribed strength. The mixture was shaken frequently for 6 hours and allowed to stand for 18 hours. It was quickly filtered after that. To prevent solvent loss, 10 ml of the filtrate was evaporated to dryness in a shallow dish made of alcohol with a tarred bottom and dried at 105°C to a consistent weight.
Total ash: 5 gm of the five samples (each weighing 1 gm) were burned at a temperature of up to 450°C in a platinum dish coated with tar until free carbon was produced. After cooling and being weighed, the amount of ash was determined. Acid insoluble ash: The insoluble material was collected in a blank filter paper, rinsed with hot water and burned to a consistent weight after all 5 samples of ash were heated for 5 minutes with 60 ml (each containing 10 ml) of diluted HCL. It was computed how much of the ash acid insoluble was.
Study using X-Ray Diffraction (XRD): The quick analytical tool known as X-Ray powder Diffraction (XRD) is used to determine the phase of crystalline materials. The finely ground, homogenised sample is used to calculate the bulk average composition. A high Resolution powder Diffractometer XRD (Rigaku) based on a (40 mA) 45 kw rotating anode X-Ray generator with curved crystal monochromater coupled with a high temperature attachment was used to characterise the powder of five ashes. Randomly oriented samples between 2 value: 1-89° were used for the ray diffractometer scans. It was conducted by the Garhwal HNBGU school of science at the central university (DOPS).
A total of 5 gm of ash (each sample being 1 gm) were kept in the groove of the X-ray diffractometer's sample holder. In order to prevent errors caused by specimens with rough surfaces, the samples' surfaces were rendered flat.
Principle: when an x-ray beam strikes a solid, the electrons that make up its atoms start to behave like tiny oscillators. These fluctuate at the same frequency as the x-rays that are being received. These scattered waves originate from electrons that move in specific directions after being grouped regularly in a crystal lattice. These waves are considered to be diffracted by the crystal plane if they experience constructive interference. Every crystalline substance scatters the x-rays in a distinct diffraction pattern that leaves a fingerprint of the atomic and molecular composition of the substance.
Applications include characterizing heterogeneous solid mixtures and crystallographic structures. It is used to determine the true condition of a chemical mixture and relative abundance. It is the sole technique available for identifying a substance's polymorphs. From a pharmacological perspective, the impact of polymorphism on solubility is particularly significant.
Phase identification of Loha Bhasam: Iron Oxide (Fe2O3) is the predominant phase in Loha Bhasma sample 3 while iron (FeS) is the minor phase in samples from the other two batches.
Scanning electron microscope: With the use of a high energy electron beam that scans the sample surface in a raster scan pattern, the Scanning Electron Microscope (SEM) creates images of the surface. An electron microscope is a type of microscope that enlightens a specimen with an electron particle beam and produces an image that is greatly enlarged. While the best light microscopes are only capable of magnifications of 2000 times, electron microscopes have significantly higher resolving power than light microscopes that rely on electromagnetic radiation. They can also achieve much higher magnifications of up to 2 million times.
Application: Morphology, topography, crystallographic information and composition.
Methods: Using two different types of parameters, the samples were put through analysis.
Evaluation using standard analytical parameters
Organoleptic characteristics:
• Varitara test
• Unam test
• Rekhapurnata test
• Nishchandratwa test
• Apunarbhavata test
• Niruttha test
Evaluation of modern parameters
• Loss on drying
• Ash value
• Ash soluble in acid
Limit test for lead: The colorimetric test method can be used to conduct a lead limit test. This is how you do it:
Materials: Test sample; known concentration of lead standard solution
• A solution of sodium sulphide.
• A solution of sodium acetate.
• A solution of ammonium oxalate.
• A solution of hydrochloric acid.
• Spirited water.
• Testing tubes.
• Graded pipettes.
• A spectroscope.
• Preparing a series of lead standard solutions with concentrations ranging from 0.1 mg/l to 0 mg/l is the first step in the procedure.
• Put 5 ml of the sample into a test tube using a pipette.
• Stir in 2 ml of sodium sulphide solution.
• Stir in 2 ml of sodium acetate solution.
• Stir in 1 ml of the ammonium oxalate solution.
• Stir in 5 ml of the hydrochloric acid solution.
• Add distilled water to the solution to a final volume of 25 ml.
• Create a blank solution by carrying out steps 2 through 7 but substituting distilled water for the sample.
• Using a spectrophotometer, calculate the absorbance of the sample and the standard solutions at 364 nm.
• Create a calibration curve by graphing the absorbance the standard solution concentration.
• Use the sample's absorbance to calculate the amount of lead present by comparing it to the calibration curve.
Interpretation: The result is reported as "not detected" or "less than the limit of detection" if the lead concentration in the sample is below the method's limit of detection. The actual lead concentration in the sample is reported as the result. It's crucial to keep in mind that this method is just one of several potential approaches to conducting a limit test for lead, and the precise approach employed will depend on elements like the kind of sample and accessible resources. Furthermore, good laboratory quality and safety to guarantee accurate and trustworthy outcomes, control methods should be adhered to.
Limit test for mercury: Utilise the Cold Vapour Atomic Absorption Spectroscopy (CVAAS) technique to conduct a limit test for mercury:
Materials: Test sample; a known concentration of the mercury standard solution.
•Solution of stannous chloride.
•A solution of hydrochloric acid.
•Solution of sodium hydroxide.
•Solution of potassium permanganate.
•Spirited water.
•Testing tubes, graduated pipettes and a CVAAS device.
•Preparing a series of mercury standard solutions with concentrations ranging from 0.1 g/l to 1.0 g/l is the first step in the procedure.
•Put 5 ml of the sample into a test tube using a pipette.
•Stir in 5 ml of stannous chloride solution.
•Stir in 5 ml of the hydrochloric acid solution.
•To make the mercury into its elemental form, place the test tube in a water bath set at 50°C to 60°C for 10 minutes.
•To correct the pH, add 5 ml of sodium hydroxide solution and thoroughly combine.
•To produce the cold mercury vapour, place the test tube in a water bath set at 50 to 60 degrees Celsius for 10 minutes.
•Inject the chilly vapour into the CVAAS device, then check the absorbance at the proper wavelength (253.7 nm for mercury).
•Draw a calibration curve by tracing the absorbance vs. the standard solution concentration.
•Calculate the sample's mercury concentration by contrasting its absorbance to the calibration curve.
Interpretation: The result is reported as "not detected" or"less than the limit of detection" if the concentration of mercury in the sample is below the method's limit of detection. The result is presented as the actual concentration of mercury in the sample if the concentration is higher than the limit of detection.
It's crucial to keep in mind that this procedure is just one of several potential approaches to conducting a limit test for mercury and the precise approach employed will rely on elements like the kind of sample and accessible resources. To achieve accurate and trustworthy results, proper laboratory safety and quality control procedures.
Limit test for arsenic: The Silver Diethyl Dithio Carbamate (SDDC) method to conduct an arsenic limit test:
Materials: The test sample.
•A standard solution of known concentration of arsenic.
•SDDC remedy.
•A solution of hydrochloric acid.
•A solution of sodium acetate.
•A solution of sodium chloride.
•Spirited water testing tubes.
•Graded pipettes.
•Spectrophotometer for atomic absorption.
Procedure
•Create a set of standard solutions with varying amounts of arsenic, ranging from 0.1 mg/l to 1.0 mg/l.
•Put 5 ml of the sample into a test tube using a pipette.
•Stir in 1 ml of the hydrochloric acid solution.
•Stir in 1 ml of sodium acetate solution.
•Stir in 1 ml of the SDDC solution.
•Stir in 2 ml of sodium chloride solution.
•Add distilled water to the solution to a final volume of 25 ml.
•Create a blank solution by carrying out steps 2 through 7 but substituting distilled water for the sample.
•Utilise an atomic absorption spectrophotometer to measure the absorbance of the sample and the standard solutions at 525 nm.
•Create a calibration curve by graphing the absorbance vs the standard solution concentration.
•Use the sample's absorbance in comparison to the calibration curve to determine the amount of arsenic present.
Interpretat on: The result is reported as "not detected" or"less than the limit of detection" if the concentration of arsenic in the sample is below the method's limit of detection.
If the concentration is greater than the detection threshold, the actual amount of arsenic in the sample is reported in the result. It's crucial to keep in mind that this method is just one of several potential approaches to conducting a limit test for arsenic and the precise approach employed will rely on elements like the kind of sample and accessible resources. To achieve accurate and trustworthy results, proper laboratory safety and quality control procedures should be followed.
Results and Discussion
Ayurvedic medicine Loha Bhasam contains iron oxide and additional natural components. Arsenic, lead and mercury pollution are possible with herbal medicines, just like with any other kind of medication. Indian regulatory agencies have established restrictions on the amount of heavy metals in Ayurvedic medicines to ensure their safety. Which are permitted in these goods. Arsenic in Ayurvedic medications is limited to 10 parts per million (ppm), but the lead and mercury limits are 2.5 ppm and 1 ppm, respectively. You can do a limit test on Loha Bhasam to check for the presence of heavy metals including arsenic, lead and mercury. A limit test is mixing a small amount of the sample with a reagent and looking for any colour alterations that might signify the presence of heavy metals. A yellow hue denotes the presence of the element arsenic. A dark colour, such as black or brown, denotes the presence of lead. Grey or black coloration denotes the presence of mercury. It is crucial to remember that Ayurvedic treatments should never replace Western medicine and should always be taken under the supervision of a trained professional (Figure 1). A healthcare expert should be consulted if you have any health issues.
Additionally, it's critical to buy Ayurvedic medications from reliable vendors who put their products through strict quality control checks to guarantee their purity and safety (Tables 2-4).
| s. no | Parameters | S-1 | S-2 | S-3 | S-4 | S-5 |
|---|---|---|---|---|---|---|
| 1 | LOD | 0.04 | 0.05 | 0.03 | 0.05 | 0.04 |
| 2 | Water soluble extractive | 0.06 | 0.05 | 0.06 | 0.03 | 0.06 |
| 3 | Total ash | 9.00% | 8.90% | 12% | 8% | 9.30% |
| 4 | Acid insoluble ash | 1.8 | 2.1 | 1.9 | 3.1 | 2.7 |
| 5 | pH | 6.9 | 7 | 7.1 | 7.2 | 7.3 |
Table 2: Physico chemical analysis of sample1 to 5.
| S. no | Bhasam company | Sample name | State | Colour |
|---|---|---|---|---|
| 1 | Divya | Batch 1st | Amorphous | Brown |
| 2 | Badhyanath | Batch 2nd | Amorphous | Reddish brown |
| 3 | Dhootapes | Batch 3rd | Amorphous | Red |
| 4 | Dabour | Batch 4th | Amorphous | Black |
| 5 | Unja | Batch 5th | Amorphous | Reddish brown |
Table 3: Samples with their state and color.
| Sample | Varna (Colour) | Niswadu (Taste) | Nischandrata (Lusterness) | Varitara (Lightness) | Nirdhuma (Fumes) | Rekhapurnata (Fineness) |
|---|---|---|---|---|---|---|
| Batch 1 | Brown | Tasteless | No luster | Positive | Mild | Positive |
| Batch 2 | Reddish brown | Tasteless | No luster | Positive | Mild | Positive |
| Batch 3 | Red | Tasteless | No luster | Positive | Mild | Positive |
| Batch 4 | Black | Tasteless | No luster | postiive | Mild | Positive |
| Batch 5 | Reddish brown | Tasteless | No luster | positive | Mild | Positive |
Table 4: Characteristics of samples.
XRD data: All batches 1 TO 5
Batch 1st
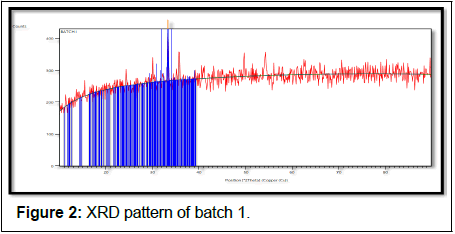
The batch 1 results show in Tables 5 and 6, Figure 2.
| Pos. (°2Th.) | Height (cts) | FWHM left (°2Th.) | d-spacing (Å) | Rel. Int. (%) |
|---|---|---|---|---|
| 33.27 (1) | 125 (16) | 0.23 (4) | 2.69059 | 100 |
| 33.36 (1) | 63 (16) | 0.23 (4) | 2.69059 | 50 |
Table 5: X-ray diffraction peak list of batch 1.
| Visible | Ref. code | Score | Compound name | Displacement (°2Th.) | Scale factor | Chemical Formula |
|---|---|---|---|---|---|---|
| * | 96-720-9375 | 27 | Ag8 (Ge3 O10) | 0 | 1.001 | Ag64.00 O80.00 Ge24.00 |
Table 6: X-ray diffraction of Ag64, O80, Ge24.
Batch 2nd
The batch 2 results show in Tables 7 and 8.
| 2theta (deg) | Intensity % |
|---|---|
| 35.64 | 100 |
Table 7: Relation between 2theta (deg) and intensity.
| Visible | Ref. code | Score | Compound name | Displacement (°2Th.) | Scale factor | Chemical formula |
|---|---|---|---|---|---|---|
| * | 01-073-0603 | 33 | Iron oxide | 0 | 0.931 | Fe2O3 |
Table 8: X-ray diffraction of Fe2O3.
Batch 3rd
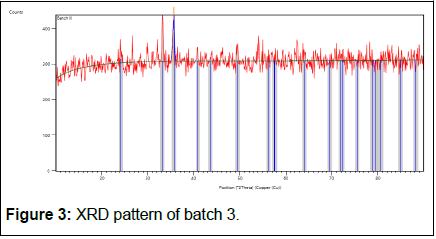
The batch 3 results show in Tables 9 and 10, Figure 3.
| Pos. (°2Th.) | Height (cts) | FWHM left (°2Th.) | d-spacing (Å) | Rel. Int. (%) |
|---|---|---|---|---|
| 35.56 (2) | 81 (11) | 0.41 (7) | 2.52261 | 100 |
| 35.65 (2) | 41 (11) | 0.41 (7) | 2.52261 | 50 |
Table 9: X-ray diffraction peak list of batch 3.
| Visible | Ref. code | Score | Compound name | Displacement (°2Th.) | Scale factor | Chemical formula |
|---|---|---|---|---|---|---|
| * | 01-073-0603 | 62 | Iron oxide | 0 | 0.999 | Fe2O3 |
Table 10: X-ray diffraction of Fe2O3.
Batch 4th
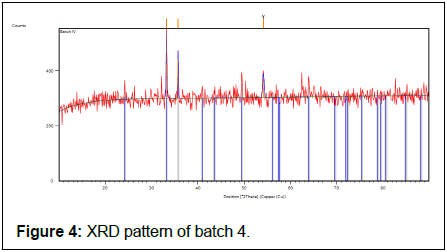
The batch 4 results show in Table 11 and Figure 4.
| Pos. (°2Th.) | Height (cts) | FWHM left (°2Th.) | d-spacing (Å) | Rel. Int. (%) |
|---|---|---|---|---|
| 33.16 (2) | 251 (186) | 0 (1) | 2.69949 | 100 |
| 33.24 (2) | 126 (186) | 0 (1) | 2.69949 | 50 |
| 35.63 (1) | 143 (25) | 0.15 (3) | 2.51748 | 56.7 |
| 35.73 (1) | 71 (25) | 0.15 (3) | 2.51748 | 28.35 |
| 54.07 (3) | 66 (14) | 0.33 (9) | 1.69467 | 26.21 |
| 54.22 (3) | 33 (14) | 0.33 (9) | 1.69467 | 13.1 |
Table 11: X-ray diffraction peak list of batch 4.
Batch 5th
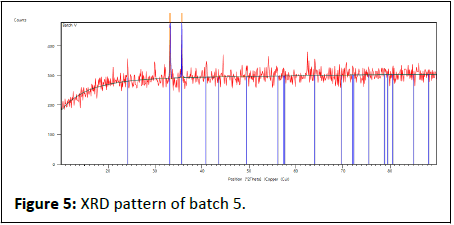
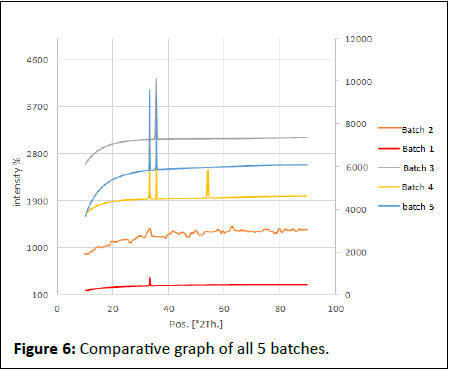
The batch 4 results show in Table 12, Figures 5 and 6.
| Pos. (°2Th.) | Height (cts) | FWHM left (°2Th.) | d-spacing (Å) | Rel. Int. (%) |
|---|---|---|---|---|
| 33.181 (9) | 170 (26) | 0.17 (3) | 2.69776 | 83.92 |
| 33.266 (9) | 85 (26) | 0.17 (3) | 2.69776 | 41.96 |
| 35.63 (2) | 203 (191) | 0.08 (9) | 2.51805 | 100 |
| 35.72 (2) | 101 (191) | 0.08 (9) | 2.51805 | 50 |
Table 12: X-ray diffraction peak list of batch 5.
FTIR: Infrared Fourier Transform Spectroscopy (FTIR) transforming Fourier analytical methods like infrared spectroscopy, sometimes referred to as FTIR analysis or FTIR spectroscopy, are used to distinguish between organic, polymeric, and, in some situations, inorganic materials. The FTIR analysis method scans test materials and examines chemical characteristics using infrared light. It is a well-known method for ensuring the quality of industrially produced materials, and it is frequently the initial stage of the material analysis process. A change in the material's composition or the presence of contamination is clearly shown by a change in the distinctive pattern of absorption bands. Visual inspection may reveal product flaws, and FTIR microanalysis is often used to pinpoint their source. This method is excellent for determining the chemical composition of larger surface regions as well as tiny particles, typically 10 to 50 microns in size.
Principle: A sample is exposed to infrared light from the FTIR instrument that ranges in wavelength from 10,000 to 100 cm-1, some of which is absorbed and some of which passes through. The sample molecules transform the absorbed radiation into rotational and/or vibrational energy. The resulting signal, which appears as a spectrum at the detector and typically ranges from 4000 cm-1 to 400 cm-1, represents the sample's molecular fingerprint. Because each molecule or chemical structure will provide a distinct spectral fingerprint, FTIR analysis is a fantastic technique for identifying specific chemicals.
Functional group/compound present
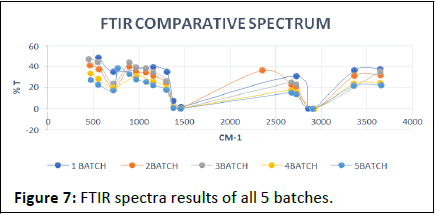
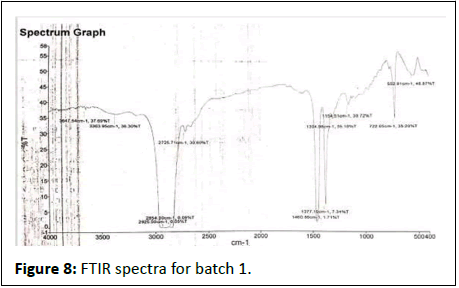
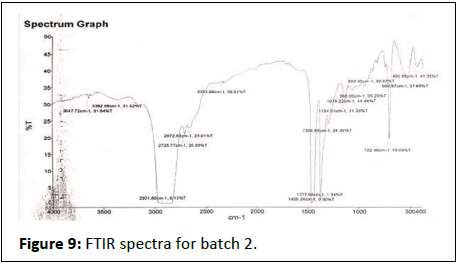
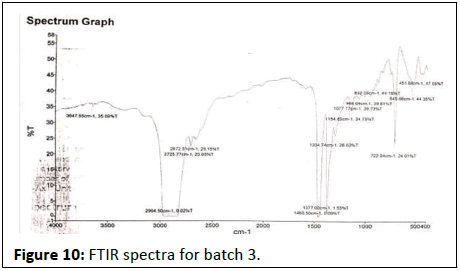
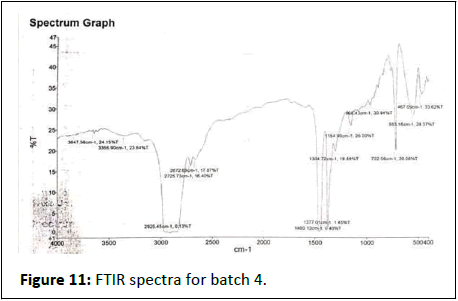
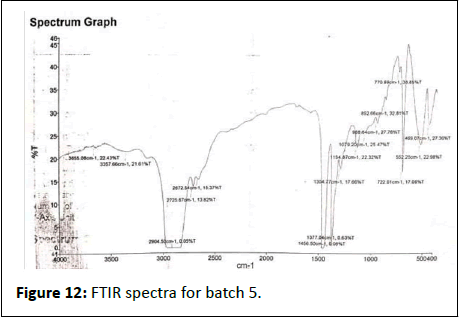
FTIR results show in Figures 7-12.
Batch 1st
• 3647-Hydroxy group (OH), amide group (NH), H2O
• 3363-Alcohol, phenol and carboxilic acid
• 2925-Carbon, hydrogen
• 2854-Methyl group (alkanes)
• 2725- Aldehyde, ketone and methylene group
• 1304-Methylene, methine
• 1154-Ester, ether, carboxilic acid
• 722-Alkene
• 552-Alkyl halide
Batch 2nd
• 3647-Hydroxy group (OH), amide group (NH), H2O
• 2.3362-Amine, hydroxyl group, alcohol phenols
• 3.2921-Alkane, aldehyde, ketones
• 4.2725-Alkane, alkenes, alkynes, formaldehyde
• 5.2672-Alkane, alkenes, alkynes
• 6.2351-Carbondioxide, isocyanate, azide
• 7.1456-Methylene, alkene, methyl
• 8.1377-Alkene, methyl
• 9.1304-Alkanes ,alkenes ,aromatic compound
• 10.1154-Alcohols, esters, ethers, carboxilice acid
• 11.1078-Alcohols,esters ,ethers, carboxilic acid
• 12.968-Alkyl group, aromatic ring
• 13.892-Alkyl group, aromatic ring
• 14.560-Chloro, bromo
• 15.460-Chlorine, bromine, iodine
Batch 3rd
•3647-Hydroxy group (OH), amide group(NH), H2O 2904-Alkane, aldehyde, ketone
• 2725-Alkyne, carbonyl, aromatic
• 2672-Alkane, alkenes, alkynes
• 1460- Alkene, aromatic, methylene
• 1377-Alkene, methyl
• 1304-Alkanes, alkenes, aromatic compound
• 1154-Alcohols, esters, ethers, carboxilice acid
• 1077-Ester,ether, alcohol, phenolic
• 968-Alkyl group, aromatic ring
• 892-Alkyl group, aromatic ring
• 722-Alkene
• 545-Halogen substitution, alkene
• 451-Alkyl halides, alkene, cyclic, aromatic
Batch 4th
• 3647-Amine groups, hydroxyl group
• 3356-Carbonyl, water
• 2925-Methyl group
• 2725-Alkyne, carbonyl, aromatic
• 2672-Alkane, alkenes, alkynes
• 1460-Alkene, aromatic, methylene
• 1377-Alkene, methyl
• 1304-Alkanes, alkenes, aromatic compound
• 1154-Alcohols, esters, ethers, carboxilice acid
• 968-Alkyl group, aromatic ring
• 722-Alkene
• 553-Metal halogens compounds, metal chlorine
Batch 5th
• 3655-Hydroxyl, amine water, alcohol, phenols
• 3357-Amines amide
• 2904-Alkane, methylene, hydrocarbon chain, alkyl group, aldehyde, carbonyl group
• 2725-Alkyne, carboxylic acid
• 2672-Aldehyde,conjugated system
• 1456-Alkene, aromatic
• 1377-Alkene, aromatic ring
• 1304-Methyl, methylene, amine amide group
• 1154-Alcohols, esters, ethers, amine amide
Conclusion
Different physicochemical characteristics of the raw materials used to prepare the Loha Bhasam, as well as those of a commercial sample, were identified. The physicochemical analysis of raw materials revealed that the results complied with the limits specified in the Indian Ayurvedic pharmacopoeia.
Numerous physicochemical parameters of the synthesized Loha Bhasam are compared to the commercial formulation of Lauh. There was no discernible difference between the outcomes of the prepared sample of Lauh and the marketed sample of Lauh for any of the standardization parameters. Using the cutting edge analytical method X-RD, developed formulation and marketed formulation were further standardized. As they were studied for the physical properties and the composition, it was discovered that the results varied. Using the Bhasma, altered the formulation's iron concentration to boost the body's ability to absorb iron or make the iron more accessible. Therefore, it is crucial to evaluate the quality and characteristics of traditional drugs like Lauh in order to further improve their quality and boost their efficacy. This was made clear by the standardization of traditional systems of medicine using modern analytical techniques. Drug standardization requires physicochemical characterization. Modern analytical techniques like SEM and EDAX were used to analyse commercial Bhasma formulations to determine the changes in composition. The use of heavy metals in Ayurvedic formulations for illness treatment and prevention is emphasised in the literature. Due to the potential for heavy metal toxicity, it is crucial to assess the contents of Ayurvedic medicines. Lead, mercury, chromium, iron, zinc, nickel, cadmium, arsenic and tungsten are examples of heavy metals used in Ayurvedic medicine after applying particular physiochemical procedures like sublimation, heating, etc. to detoxify the metals and prevent their toxicity. Ayurvedic medicines can significantly vary due to improper preparation and the use of subpar raw components. Although Bhasma is an important component of Ayurveda, the inability to evaluate the quality of Bhasma within the framework of conventional medicine poses a significant obstacle to its growth, development, and application as a preventive and curative medicine. Standardisation of the synthesis processes is necessary to avoid the availability of Bhasma products of subpar quality. Additionally, it will aid in improving the quality, safety and effectiveness of these metals based treatments to fine tune the application of ancient and modern medical systems, such as Ayurveda, to enhance the quality of life for all people.
5-arylidene-3-amino-Arylthiazolidin-4-ones were combined and isolated in various yields. The answer is then all considerations under the properties of substituents that give electrons or provide electrons are aldehydes. Structural safety was achieved by IR, NMR and X-ray diffraction. These chiral particles have large energy components for delocalization. This is indicated by the varying frequency of the carbonyl content, which depends on the idea of the arylidene component. These mixtures have all the properties of good contraindications for application in the field of solar oriented cells and nonlinear optics.
References
- Palbag S, Mondal S, Bardhan T, Gautam DN (2016) Comparative physico-chemical validation between Arsenic-based Indian traditional drugs Haratal Bhasma and Rasamanikya. J Ayu Herb Med 2:43-48
- Paudel R, Karn G, Aryal G, Giri J, Adhikari R, et al. (2022) Synthesis, characterization, biological study of synthesized Lauha Bhasma. J Nepal Chem Soc 43:14-15
- Tiwari MK, Singh A, Khooha A, Goutam UK (2023) Structural investigation of Ayurveda Lauha (Iron) Bhasma. J Ayurveda Integr Med 14:100690
[Crossref] [Google Scholar] [PubMed]
- de Zoysa HPE, Jayaweera C, Dassanayake MRP. Characterization and Analysis of Bhasma, International Journal of Recent Research in Life Sciences (IJRRLS).
- Bambagiotti-Alberti M, Bartolucci G, Bruni B, Coran SA, Di Vaira M (2009) Physico-chemical and structural characterization of diacerhein. J Pharm Biomed Anal 49:1065-1069
[Crossref] [Google Scholar] [PubMed]
- Sun RC, Tomkinson J, Wang YX, Xiao B (2000) Physico-chemical and structural characterization of hemicelluloses from wheat straw by alkaline peroxide extraction. Polymer 41:2647-2656

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences