Acute and Sub-chronic Toxicity Studies of Methanol Leaf Extract of Cassia singueana F. (Fresen) in Wistar Rats
Yusuf Ibrahim Alkali, Abdulgafar O Jimoh and Umar Muhammad
DOI10.21767/2472-0151.100038
Yusuf Ibrahim Alkali1*, Abdulgafar O Jimoh2 and Umar Muhammad3
1Faculty of Pharmaceutical Sciences, Department of Pharmacology and Toxicology, Usmanu Danfodiyo University, Sokoto, Nigeria
2Department of Pharmacology and Therapeutics, College of Health Sciences, Usmanu Danfodiyo University, Sokoto, Nigeria
3Department of Histopathology, Usmanu Dan Fodiyo University, Sokoto, Nigeria
- *Corresponding Author:
- Yusuf Ibrahim Alkali
Faculty of Pharmaceutical Sciences
Department of Pharmacology and Toxicology
Usmanu Danfodiyo University, Sokoto, Nigeria
Tel: 08069048356
E-mail: galilie050@gmail.com
Received Date: October 29, 2018; Accepted Date: November 30, 2018; Published Date: December 08, 2018
Citation: Alkali YI, Jimoh AO, Muhammad U (2018) Acute and Sub-chronic Toxicity Studies of Methanol Leaf Extract of Cassia singueana F. (Fresen) in Wistar Rats. Herb Med Vol.4 No.2:06.
Copyright: © 2018 Alkali YI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The aim of this study was to evaluate the toxicity profile of methanol leaf extract of Cassia singueana (fabaceae) in wistar rats. Fresh leaf of the plant was shade dried and pulverized to powder after which it was subjected to maceration with methanol and 9.2% yield was obtained at end of the extraction process. An acute toxicity study was carried out using lork’s method and sub-chronic toxicity study was also carried out using OECD guideline. In the acute toxicity study, the first phase of the method, nine (9) wistar rats were randomly divided into three groups (1-3) of three (3) animals each and they received 10,100 and 1000 mg/kg (po) Cassia singueana methanol leaf extract respectively and in the second phase four (4) animals were divided into four groups of one animal each which received 1200, 1600, 2900 and 5000 mg/kg (po) of extract respectively. In the sub-chronic toxicity study twenty (20) animals were divided randomly into four group each containing five animals, the first, second, third and fourth group received distilled water, 200, 400 and 800 mg/kg extract (po) respectively. There was no mortality observed in phase 1 of acute toxicity and in phase II there was mortality at 2900 and 5000 mg/kg group. The oral administration of methanol leaf extract of Cassia singueana for 28 days did not produce significant alteration in the renal function indices. The histological section of the rats indicates normal glumeruli and regular renal tubules. Also, this study reveals no significant increase in the level of liver enzyme. Also, the histology section reveals normal central vein and regular hepatocyte separated by sinusoid. The haematopoetic indices reveal no destruction of Red Blood Cell and no change in the rate of production of the RBCs and other haematopoetic parameters. The histology of the rat’s brain revealed no deleterious effect. It can be concluded that, the methanol leaf extract of Cassia singueana is relatively non- toxic.
Keywords
Methanol leaf extract; Cassia singueana; Subchronic toxicity; Alkaline phosphatase
Introduction
Toxicity is the extent to which a substance can harm a living organism. Toxicity studies are performed to determine the safety or hazard elicited by substances such as pharmaceuticals, laboratory chemicals and consumer products. Toxicity studies can be acute, sub-chronic or chronic. Acute toxicity is the noxious effect in an organism through a single dose exposure. Sub-chronic toxicity study is aimed to evaluate the ability of a test substance to cause noxious effects after repeated exposure to the living organism.
The experimental animals are administered daily doses of the test substance to calculate the no observed adverse effect level and determine whether one or more organ/system is mildly or severely affected following exposure period of between five to ninety days. Chronic toxicity study is aimed to evaluate the ability of a test substance to cause noxious effects over an extended period of continuous exposure, sometimes lasting for the entire life of the organism.
Cassia singueana a small tree shrub 1-6 m high with spreading rounded open crown 2 m in diameter. Trunk to 15 cm across, with dark grey, rough bark irregularly longitudinally fissured; slash light brown, yellow within. Stems of branchlets faintly longitudinally ridged to teret, young apice densely pubescent with curled white hairs interspersed among minute ones forming an underlayer, becoming sparsely pubescent and glabrous as bark develops.
Leaves: petiole and rhachis 4-30 cm long. Stipules subulate, A ± 5 mm long, 3 mm wide, caducious; petiole 1.5-5 cm long including basal pulvinus, petiole gland lacking, rhachis channeled, with stalked, fusiform to elliptic, deciduous gland between each pair of leaflets, sometimes excepting the terminal leaflet [1]. The ethyl-acetate and ethanol root bark extract of Cassia singueana show promising antioxidant properties both in vivo and in vitro [2]. The Methanol root extract of Cassia singueana was also reported to have hepatoprotective and hypolipidemic effects in rats [3]. Cassia singueana methanol leaf possessed antiulcer effect [4]. It was also reported by Harami et al. [5] that Cassia singueana has antifungal activity. Cassia singueana has many medicinal uses throughout Africa. A hot water infusion of the leaves is drunk, and the warm leaves are applied as a compress to treat fever. The leaf sap is drunk to cure malaria [6]. The leaves in decoction or infusion or as dried powder are applied to wounds caused by leprosy and syphilis. An infusion of the leaves is applied as eye drops to cure conjunctivitis [6]. Extracts of the stem bark are taken to cure stomach complaints.
Like the leaves, the stem bark is used to treat skin disorders and malaria. An infusion of the flowers is used as an eye lotion. The fruit pulp soaked in water and cooked with a staple food is eaten by lactating women as it is considered lactogenic. The roots are used to treat venereal diseases, stomach complaints and as a purgative. The roots are also used to cure impotence caused by diabetes. The ash of burnt roots is eaten mixed with porridge to cure abdominal pain. Leaves stem and root barks are used as anthelminthic and to treat bilharzia. It is also used as a cooked vegetable in Malawi and Tanzania [6]. Cassia singueana has been shown after research to have medicinal properties.
Materials and Methods
Collection of plant material and preparation of extract
The plant was collected in Dange shuni Local Government Area of Sokoto in January, 2018. Sokoto State Nigeria. The plant was identified and authenticated in the Department of Pharmacognosy and Ethnopharmacy, Faculty of Pharmaceutical Sciences Usmanu Danfodiyo University Sokoto and was deposited in the herbarium. The leaves of Cassia singueana were shade dried. The dried leaves was pulverized to powder using wooden pestle and mortar and then 500 g of the dried powder was weighed and subjected to cold maceration for 72 hrs with 95% methanol as the solvent of extraction, the mixture was allowed to stand for 5 hours and was then shaken periodically. Whatman filter paper was used for filtration of the mixture and the extract was concentrated on water bath at 40°C. The dried extract was transferred to airtide container for storage.
Experimental animals
Wistar rats of both sexes weighing (120-150 g) was used in this study. The animals were maintained in a cage well ventilated with access to food and water ad libitum. All animal experimental protocols were conducted in compliance with human animal care standards outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Acute toxicity study
The method described by Lorke [7] was used in this study. This test was conducted in phases. Initial phase, nine animals of either sexes were randomly divided into three groups with three mice each. First, second and third group received 10, 100 and 1000 mg/kg of the extract and were observed for 24 hrs for mortality or any sign of toxicity.
In the second phase, we used four mice, one per each group and dose with 1200, 1600, 2900 and 5000 mg/kg. The same procedure was also used for rats. The LD50 was determined by calculating the geometric mean of the highest dose that the rats survive and lowest dose that killed the mice.
28 Days sub-chronic toxicity studies
The Organization for Economic and Community Development (OECD 407) method was adopted for the study: Total number of animals: twenty-four of either sex were randomly divided into four groups of six rats each (3 male and 3 female). First group received 10 ml/kg distilled water orally. The second, third and fourth received 200, 400 and 800 mg/kg respectively daily for 28 days orally.
The rats access food and water throughout the duration of the experiment (28 days). Observation was done on daily basis for general symptoms of toxicity and mortality. Weights of the animals were taken weekly. On the 29th day, under light chloroform anesthesia the animals were sacrificed. Blood samples were collected for haematological and biochemical tests. Histopathological examination was done on heart, liver, kidney and brain.
Results
Acute toxicity studies
There was no mortality observed in the first phase of acute toxicity study, while in phase mortality was observed in 2900 and 5000 mg extract treated group as stated in Tables 1a and 1b.
| Dose (mg/kg) PO | Number of Animals dead/used | % Mortality |
|---|---|---|
| Phase I | ||
| 10 | 0/3 | 0 |
| 100 | 0/3 | 0 |
| 1000 | 0/3 | 0 |
| Phase II | ||
| 1000 | 0/1 | 0 |
| 1600 | 0/1 | 0 |
| 2900 | 01-Jan | 100 |
| 5000 | 01-Jan | 100 |
Table 1a: Result of Oral Acute toxicity studies of methanol Leaf extract of Cassia singueana on mice.
| Dose (mg/kg) PO | Number of Animals dead/used | % Mortality |
|---|---|---|
| Phase I | ||
| 10 | 0/3 | 0 |
| 100 | 0/3 | 0 |
| 1000 | 0/3 | 0 |
| Phase II | ||
| 1000 | 0/1 | 0 |
| 1600 | 0/1 | 0 |
| 2900 | 0/1 | 0 |
| 5000 | 0/1 | 0 |
Table 1b: Result of oral acute toxicity studies of methanol leaf extract of Cassia singueana on rats.
Effect of methanol leaf extract of Cassia singueana on haematological indices following 28 days sub-chronic oral treatment in wistar rats
After twenty-eight days oral administration of methanol leaf extract of Cassia singueana, there was significant (p<0.05) decrease in white blood cell count in all the extract treated groups (200, 400 and 800 mg/kg).
There was no significant decrease in other hematological parameters such as red blood cell count, haemoglobin, platlets, packed cell volume, mean cell volume and haematocrit (Table 2).
| Treatment/Dose (mg/kg) | WBC (× 103 µL) | RBC (× 103 µL) | HGB (g/dl) | HCT (%) | PCV (%) | PLT (µL × 103) | MCV (fl) |
|---|---|---|---|---|---|---|---|
| Distilled water | 21.71 ± 1.60 | 5.98 ± 0.43 | 11.92 ± 0.73 | 37.25 ± 1.48 | 35.77 ± 2.19 | 630.50 ± 2.10 | 61.68 ± 0.49 |
| 200 CSE | 15.85 ± 0.70* | 5.27 ± 0.41 | 11.37 ± 0.43 | 33.80 ± 140 | 34.12 ± 1.29 | 620.00 ± 4.14 | 60.22 ± 1.81 |
| 400 CSE | 11.92 ± 0.65* | 5.61 ± 0.24 | 8.55 ± 2.28 | 32.37 ± 0.76 | 33.15 ± 0.75 | 510.00 ± 5.49* | 57.90 ± 2.05 |
| 800 CSE | 11.00 ± 0.61* | 5.95 ± 0.28 | 12.92 ± 0.58 | 33.10 ± 1.92 | 38.77 ± 1.75 | 557.25 ± 4.27 | 57.07 ± 1.76 |
| Data expressed as Mean ± SEM, SEM=Standard Error of Mean n=6, “*” p<0.05, WBC=White Blood Cells, RBC=Red Blood Cells, HCT=Haematocrit, HGB=Haemoglobin, PCV=Packed Cell Volume, PLT=Platelet, MCV=Mean Cells Volume, CSE=Cassia singueana methanol leaf extract. Dunnet post-hoc test. | |||||||
Table 2: Effect of methanol leaf extract of Cassia singueana on haematological indices following 28 days sub-chronic oral treatment in Wistar Rats.
Effect of methanol leaf extract of Cassia singueana on renal function indices following 28 days sub-chronic oral treatment in wistar rats
There was significant (p<0.05) decrease in serum urea and sodium at dose of 200 mg/kg, while in 400 mg/kg extract treated group we noted significant (p<0.05) decrease in serum urea only. In 800 mg/kg extract treated group there was no significant decrease in all the renal function indices (Table 3).
| Treatment/Dose (mg/kg) | Urea (mmol) | Creatinine (mmol) | Na+ (mmol) | K+ (mmol) | Cl- (mmol) | HCO3 (mmol) |
|---|---|---|---|---|---|---|
| Distilled water | 7.82 ± 0.22 | 0.95 ± 0.13 | 133.25 ± 1.88 | 4.32 ± 0.19 | 90.50 ± 2.25 | 25.50 ± 1.32 |
| 200 CSE | 5.50 ± 0.21* | 0.77 ± 0.08 | 132.50 ± 2.53 | 5.10 ± 0.10 | 94.75 ± 2.05 | 23.75 ± 1.37 |
| 400 CSE | 5.27 ± 0.17* | 0.75 ± 0.06 | 134.50 ± 2.90 | 4.30 ± 0.10 | 90.50 2.78 | 26.50 ± 1.84 |
| 800 CSE | 7.42 ± 0.22 | 1.07 ± 0.85 | 134.75 ± 1.97 | 4.45 ± 0.21 | 91.25 ± 4.17 | 23.50 ± 1.70 |
| Data expressed as Mean ± SEM, SEM=Standard Error of Mean n=6, “*” p<0.05, Na+=Sodium ion, K+=Potassium ion, Cl-=Chloride ion, HCO3=Bicarbonate, CSE=Cassia singueana methanol leaf extract. Dunnet Post-hoc test. | ||||||
Table 3: Effect of methanol leaf extract of Cassia singueana on renal function indices following 28 days sub-chronic oral treatment in wistar rats.
Effect of methanol leaf extract of Cassia singueana on liver function indices following 28 days sub-chronic oral treatment in wistar rats
There was no significant decrease in all the liver function parameters following 28 days oral administration of (200, 400 and 800 mg/kg) methanol leaf extract of Cassia singueana (Tables 4 and 5).
| Treatment/Dose (mg/kg) | T.B (mg %) | D.B (mg %) | ALK (m/l) | AST (m/l) | ALT (m/l) | T.P (g/l) | Albumin (g/l) |
|---|---|---|---|---|---|---|---|
| Distilled water | 0.77 ± 0.06 | 0.30 ± 0.04 | 87.75 ± 2.56 | 58.00 ± 3.08 | 75.50 ± 2.10 | 65.25 ± 2.56 | 40.50 ± 1.04 |
| 200 CSE | 0.75 ± 0.06 | 0.15 ± 0.01 | 86.25 ± 2.42 | 62.00 ± 1.58 | 75.50 ± 2.32 | 71.50 ± 3.12 | 38.75 ± 2.49 |
| 400 CSE | 0.87 ± 0.11 | 0.27 ± 0.04 | 86.25 ± 2.42 | 57.25 ± 2.42 | 67.75 ± 3.79 | 61.25 ± 1.10 | 40.25 ± 2.01 |
| 800 CSE | 0.95 ± 0.06 | 0.95 ± 0.06 | 83.75 ± 6.30 | 64.50 ± 2.17 | 71.75 ± 2.68 | 69.75 ± 3.75 | 41.00 ± 1.08 |
| Data expressed as Mean ± SEM, SEM=Standard Error of Mean n=6, T.B=Total Bilirubin, D.B=Direct Biluribin, ALK=Alkaline Phosphatase, AST=Aspartase Transaminase, ALT=Alanine Transaminase, T.P=Total Protein, CSE=Cassia singueana methanol leaf extract. Dunnet Post-hoc test. | |||||||
Table 4: Effect of methanol leaf extract of Cassia singueana on liver function indices following 28 days sub-chronic oral treatment in wistar rats.
| Treatments/Dose (mg/kg) | Week 1-mean weight (g) | Week 2-mean weight (g) | Week 3-mean weight (g) | Week 4-mean weight (g) |
|---|---|---|---|---|
| Distilled water | 185.40 ± 6.16 | 202.20 ± 6.02 | 209.80 ± 5.19 | 219.20 ± 4.06 |
| 200 CSE | 169.80 ± 2.65 | 186.00 ± 6.40 | 193.80 ± 7.49 | 215.20 ± 7.31 |
| 400 CSE | 175.80 ± 8.94 | 181.80 ± 10.41 | 199.00 ± 14.65 | 212.80 ± 12.76 |
| 800 CSE | 182.20 ± 4.34 | 198.80 ± 5.73 | 207.60 ± 6.98 | 224.00 ± 5.73 |
| CSE=Cassia singueana methanol leaf extract. | ||||
Table 5: Effect of methanol leaf extract of Cassia singueana on body weight changes following 28 days sub-chronic oral treatment in wistar rats.
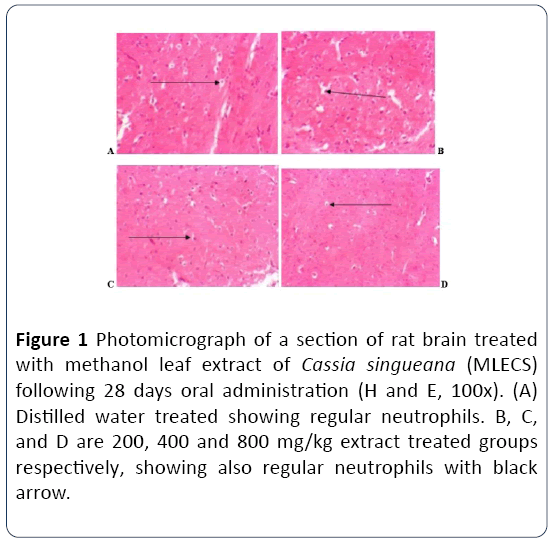
Effect of methanol leaf extract of Cassia singueana on histology of brain following 28 days oral administration in rats
The histology of the brain sections of rats following 28 days oral administration of methanol leaf extract of Cassia singueana reveals well preserved neutrophils in both the control and extract treated groups (Figure 1).
Figure 1: Photomicrograph of a section of rat brain treated with methanol leaf extract of Cassia singueana (MLECS) following 28 days oral administration (H and E, 100x). (A) Distilled water treated showing regular neutrophils. B, C, and D are 200, 400 and 800 mg/kg extract treated groups respectively, showing also regular neutrophils with black arrow.
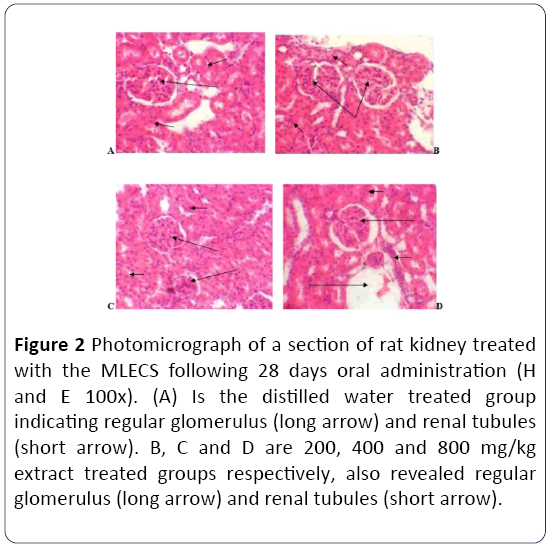
Effect of methanol leaf extract of Cassia singueana on histology of the kidney following 28 days oral administration in rats
The result indicated regular glomerulus (long arrow) and renal tubules (short arrow) in control group and also similar features were observed in the extract treated groups (Figure 2).
Figure 2: Photomicrograph of a section of rat kidney treated with the MLECS following 28 days oral administration (H and E 100x). (A) Is the distilled water treated group indicating regular glomerulus (long arrow) and renal tubules (short arrow). B, C and D are 200, 400 and 800 mg/kg extract treated groups respectively, also revealed regular glomerulus (long arrow) and renal tubules (short arrow).
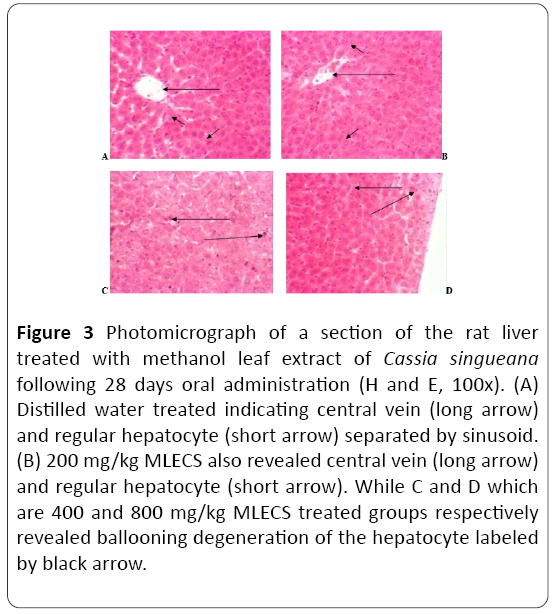
Effect of methanol leaf extract of Cassia singueana on histology of the liver following 28 days oral administration in rats
The Liver section of control group and 200 mg/kg extract treated group reveals central vein (long arrow) and regular hepatocyte (short arrow) separated by sinusoid. H and E × 100. While in the 400 and 800 mg/kg extract treated groups reveals ballooning degeneration of the hepatocyte labeled by black arrow (Figure 3).
Figure 3: Photomicrograph of a section of the rat liver treated with methanol leaf extract of Cassia singueana following 28 days oral administration (H and E, 100x). (A) Distilled water treated indicating central vein (long arrow) and regular hepatocyte (short arrow) separated by sinusoid. (B) 200 mg/kg MLECS also revealed central vein (long arrow) and regular hepatocyte (short arrow). While C and D which are 400 and 800 mg/kg MLECS treated groups respectively revealed ballooning degeneration of the hepatocyte labeled by black arrow.
Discussion and Conclusion
In the oral acute toxicity study of the plant extract we calculate the median lethal dose to be 2154 mg/kg in mice and >5000 mg/kg in rats. According to Dietrich Lorke, 1 mg/kg is considered highly toxic, 10 mg/kg is considered toxic, 100 mg/kg is moderately toxic, 1000 mg/kg is slightly toxic, and 5000 mg/kg is considered not toxic [7]. Our result indicates that large dose of the extract can be toxic and low doses are relatively safe. Mortality was only observed in phase where high doses were employed. According to the American Society for testing safety of Materials, any chemical substance with LD50 less than 2000 mg/kg/oral route but greater than 1000 mg/kg/oral could be considered to be slightly toxic.
In the 28 days sub-chronic toxicity study, there were no behavioral or observable toxic effects as a result of administering the extract for the duration of the study. There was no significant change in body weight compared with control group and extract treated group throughout the period of the study. The normal weight increment observed from day one to 2h day of the study was due to normal physiological changes in rats such as, food intake, water intake and metabolic processes in their systems. A decrease in body weight of an animal after certain exposure to a substance for a period of time is indication of harmful nature of that substance [8].
Metabolic reactions in the body are largely regulated by the liver and kidney. The renal system is largely responsible for excretion of waste product out of the body. The liver detoxifies substances that are harmful to the body while the kidney helps in maintaining homeostasis by reabsorbing vital electrolytes and excretion of waste products.
Urea and creatinine are the major indicators of renal toxicity. Serum urea accumulation is used as the acute marker, while serum creatinine accumulation is used in detecting chronic renal toxicity [9]. In this study, the oral administration of Cassia singueana extract for 28 days did not produce significant alteration in the renal function indices. The histological section of the rats indicates normal glumeruli and regular renal tubules. A similar finding was also reported by Ezejindu et al. [10] who assayed the reno-protective effects of Moringa oleifera leaf extract on the kidneys of adult Wistar rats and observed no alteration in the renal function indices and no tissue lesion in the histopathology study.
The degree of liver damage induced by a chemical substance can be evaluated by determining the level of biochemical markers of the liver function such as Aspertate transaminase (AST), Alanine transaminase (ALT), Alkaline phosphatase (ALP). ALP is located in the cytoplasm and is release into circulation after cellular hepatic damage. ALT and AST are also enzymes released as a result of liver injury, especially damage to mitochondria of liver cells. Elevation of level of these enzymes can be an indication of cellular damage, leakage and loss of functional integrity of hepatic cell membrane. The result of this study reveals no significant differences in the level of liver enzyme when Cassia singueana extract treated groups are compared with the control group. Also, the histology section reveals normal central vein and regular hepatocyte separated by sinusoid, however in the highest dose of Cassia singueana group (800 mg/kg) some early signs of inflammation manifest as ballooning. This indicates may be on chronic dosing longer than 28 days it can cause liver inflammation. Cassia singueana methanol leaf extract can be relatively safe at low dose; however, contradicting findings was reported by Peter et al., who used different plant and discovered an increase in serum concentration of ALT and ALP. The histological study of the harvested liver showed hepatocytes degeneration and periportal inflammations indicating alteration in the normal physiological status of the liver [11].
The hematopoietic physiology is very sensitive to toxins, hence values obtained after exposure of an animal to toxic compounds can be used to evaluate the pathological or physiological status of the test animal [12].
After 28 days administration of different doses (200, 400 and 800 mg/kg body weight) of Cassia singueana methanol leaf extract there was no significant difference in RBC and indices relating to it (Hb, PCV and MCV) throughout the experimental period is an indication that, there was no destruction of Red Blood Cell and no change in the rate of
production of the RBCs. This also reveals that the extract of Cassia singueana does not stimulate the release of erythropoietin from the renal system, which is humoral regulator of RBC production [13].
Also, Tohti et al. [14] has reported decrease in the white blood cell count (WBC) due to the presence of some phytochemicals such as Saponins and Cardiac Glycosides [14]. This decrease may also suggest the extract’s inhibitory effect on white blood cell production, possibly by decreasing thrombopoietin secretion. White Blood Cells function mainly to fight infection and produce, transport antibodies to various body compartments. Also, the significant decrease in platelets at dose of 400 mg/kg Cassia singueana extract was not consistent with the higher dose (800 mg/kg), hence indicating this change could be coincidental and not as a result of our extract intervention [15].
The histology of the rat’s brain revealed no deleterious effect. Regular neutrophils were observed when compared with the control group indicating no his pathological changes in the brain tissue throughout the experimental period. These observations are suggesting that, the Cassia singueana methanol leaf extract has no effect on architecture and structure of the rat’s brain.
References
- Roux JP (2003) Evaluation of South African flora. African National Biodiversity Institute, Compton Herberium of the Useful Plant.
- Ibrahim MA, Koorbanally NA, Islam MS (2013) In vitro anti-oxidative activities and GC-MS analysis of various solvent extract of Cassia singueana parts. Acta Pol Pharm 70: 709-719.
- Ottu OJ, Atawodi SE, Oyinke E (2013) Antioxidant, hepatoprotective and hypolipidemic effects of methanolic root extract of Cassia singueana in rats following acute and chronic carbon tetrachloride intoxication. Asian Pac J Trop Med 6: 609-615.
- Ode OJ, Asuzu OV (2011) Investigation of Cassia singueana leaf extract for antiulcer effects using ethanol-induced gastric ulcer Model in rats. International Journal of Plant, Animal and Environmental Sciences 2: 1.
- Harami MA, Abeyeh OJ, U-Ibok NNE, Kafu SE (2006) Antifungal activity of extracts of some Cassia, Detarium and Ziziphus species against dermotophyte. Natural Product Radiance 5: 357-360.
- Gaby HS, Gabriella HS, Gurib-Fakim A (2008) Medicinal plants. Natural Product Radiance 2: 1.
- Lorke DD (1983) A new approach to acute toxicity testing. Arch Toxicol 54: 275-283.
- Petterino C, Argentino-Storino A (2006) Clinical chemistry and Haematology historical data in controlled Sprague-Dawely rats from preclinical toxicity studies. Pharmacognosy, pp: 88-89.
- Borges LP, Borges VC, Moro AV, Nogueira CW, Rocha JB, et al. (2005) Protective effect of diphenyl diselenide on acute liver damage induced by 2-nitro propane in rats. Toxicology 210: 1-8
- Ezejindu DN, Udemezue OO, Akingboye AJ (2014) Renoprotective effects of moringa oleifera leaf extract on the kidneys of adult wistar rats. American Journal of Engineering Research 3: 157-161.
- Peter A, Njoku B, Kyrian UN (2015) Effects of ethanolic extract of Cyperus esculentus (tiger nut) on some liver function parameters using albino wistar rats. Eur J Pharm Med Res 2: 1705-1715.
- Abubakar K, Danjuma NM, Maiha BB, Anuka JA, Yam MF, et al. (2015) A 28 day oral toxicity study of Pseudocedrela kotchyi methanol extract in sprague- dawley rats. European Journal of Medicinal Plants 10: 1-11.
- Sanchez-Elsner T, Ramirez JR, Rodriguez-Sanz F, Varela E, Bernabew CLM, et al. (2004) A cross talk between hypoxia and TGF- beta onchestrates erythropoietin gene regulation through SPI and smads. J Mol Biol 36: 9-24.
- Tohti I, Tursun M, Umar A, Turdi S, Imin H, et al. (2006) Aqueous extract of Ocimum basilicum L. (sweet basil) decrease platelet aggregation induced by ADP and thrombin in vitro and rats arterio-veneous shunt thrombosis in vivo. Thrombosis Research 118: 733-739.
- OECD (2008) Test guideline 452. chronic toxicity studies. In: Draft OECD 76 guideline for testing chemicals 2: 1.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences