Melissa officinalis Extract Inhibits Herpes Simplex Virus-I Glycoprotein B Interaction with Heparin Sulfate
Karen Denzler L, Trung Huynh P, Bertram Jacobs L and Jeffrey Langland O
DOI10.21767/2472-0151.100014
Karen Denzler L1, Trung Huynh P1, Bertram Jacobs L1 and Jeffrey Langland O1,2*
1Biodesign Institute, Arizona State University, Tempe, Arizona 85287-5401, USA
2Southwest College of Naturopathic Medicine, Research Department, Tempe, AZ 85282, USA
- *Corresponding Author:
- Jeffrey Langland O
Southwest College of Naturopathic medicine
Research Department Tempe
Arizona 85252, USA
E-mail: j.langland@scnm.edu
Received date: April 14, 2016; Accepted date: April 25, 2016; Published date: April 30, 2016
Abstract
Herpes simplex virus 1, HSV1, is the primary cause of herpes labialis in humans. Drugs like acyclovir and its derivatives are available for treatment, but with increased use and the number of immune compromised patients, the development of resistance to these drugs is increasing. Extracts of the botanical, Melissa officinalis, have previously been reported to contain antiviral activity toward HSV1. Our initial studies confirmed earlier results that constituents of Melissa officinalis interacted directly with the virus and inhibited HSV1 binding to cells during the initiation of infection. Further studies demonstrated that a component in Melissa officinalis bound specifically to the viral glycoprotein B. Virion structure was shown to be stable at low concentrations of Melissa officinalis, however at a ten-fold higher concentration than that which inhibited binding, virion structure was completely disrupted suggesting a second, virucidal, mode of inhibition. Melissa officinalis was shown to inhibit other alpha herpes viruses as well as having intermediate inhibitory activity against other viruses from the adenoviridae, poxviridae, papovaviridae, and rhabdoviridae families.
Keywords
Herpes simplex; Melissa; Lemon balm; Botanical; Heparin sulfate
Introduction
Herpes simplex virus type 1 (HSV1) is a member of the Herpesviridae family that primarily causes herpes labialis (cold sores) in humans. HSV1 also causes facial, pharyngeal, ocular, central nervous system infections, and in rare cases, encephalitis. It is seroprevalent in almost 58 percent of the US population and increases in prevalence with age [1,2]. Worldwide an estimated 60-95 percent of adults are infected with HSV1 [3]. Although HSV1 infection occurs predominantly at oral sites, an increase in infection at genital sites has been reported [1].
HSV1 infection is transmitted through mucocutaneous sites via direct contact with a lesion or with body fluids like saliva, respiratory droplets, or genital secretions [3,4]. Primary infection occurs in epithelial cells of the oral mucosa or lips and can be either asymptomatic or cause erythymatous vesicles in the dermis and epidermis which is followed by establishment of latency in the innervating sensory neurons [5-9]. HSV1 is transported to the cell bodies of the neuron where latency is established, often in the trigeminal or dorsal root ganglia [4,10]. During latency, the viral lytic genes are repressed and the viral genomes are maintained as circular, extrachromosomal episomes. Periodically, the viral genomes in some of the neurons reactivate and virus travels down the axon to the original site of infection where it can cause recurrent disease resulting in skin ulcerations and pain [4,11].
Current treatment toward HSV1 infection typically uses acyclovir and the derivatives, famciclovir and valacyclovir [1,12]. Acyclovir is a guanine nucleoside analog that is converted by the viral thymidine kinase to acyclovir monophosphate. Cellular kinases add two more phosphate groups to generate acyclovir triphosphate that competitively inhibits the viral DNA polymerase and is incorporated into the viral DNA during genome replication resulting in chain termination [12]. The effectiveness of these drugs can be limited in patients with compromised immune systems or in those experiencing chronic HSV infections. In these cases, the potential for the virus developing drug resistance is higher [13,14]. In addition, these drugs can have side effects that include nausea, diarrhea, and vomiting. Drugs like foscarnet, a pyrophosphate analog that binds the viral DNA polymerase, and cidofovir, a nucleotide analog that does not require phosphorylation by the viral thymidine kinase, can be used when acyclovir resistance has occurred, although these drugs display reduced bioavailability or nephrotoxicity, respectively [15-18].
For the past half a century, the US population has been increasingly seeking alternative therapies to chronic diseases [19-21]. A study in 2002 estimated 19 percent of adults used a botanical therapy to treat illnesses or conditions [22]. Given that herpes virus infection results in lifelong persistence and is associated with recrudescence in many patients, a number of plant extracts and oils have been analyzed for therapeutic potential and include members of the Lamiaceae family: lemon balm, peppermint, prunella, rosemary, sage, and thyme; as well as birch bark, Gynura, Manuka, tea tree, eucalyptus, ginger, thyme, hyssop, sandalwood, and propolis [23-32]. These botanical may offer alternative therapies as well as the potential for isolation of novel, efficacious active compounds.
Melissa officinalis, also known as lemon balm, has been prepared as both aqueous extracts or essential oils and tested for antiherpes activity in vitro [23,33,34]. Either type of preparation displays inhibitory activity toward HSV1 and an aqueous extract was shown to inhibit acyclovir-resistant strains of HSV1 [34]. In addition, the use of a topical cream made from lemon balm in the treatment of herpes infection of the skin shows a significant reduction in the size of lesions by day 2 of treatment [35].
Infection by HSV1 is mediated by binding to specific molecules on cells using a number of virus-encoded glycoproteins that are present in the virus envelope. Initial binding occurs with glycoprotein C (gC) and glycoprotein B (gB) which bind to the cell surface glycosaminoglycans, heparan sulfate and chondroitin sulfate, or by the recently described interaction between gC and the scavenger receptor, MARCO [36-39]. Glycoprotein D (gD) then binds to entry receptors, nectin-1, nectin-2, HVEM, or 3-O-sulphated heparan sulfate and undergoes a conformational change that facilitates interactions with viral glycoproteins gB, gH, and gL [40-44]. The four glycoproteins, gD, gB, gH, and gL, are required for membrane fusion and release of the viral particle into the cytoplasm at the external plasma membrane or from endocytic vesicles [45,46].
Aqueous extracts of Melissa officinalis have been shown to inhibit binding of HSV1 virions to the cell [23,34]. Given the historical use and growing body of evidence, this study sought to further examine the antiviral activity of Melissa officinalis towards HSV1 to better delineate the mechanism of action of the whole botanical extract.
Materials and Methods
Cell lines and virus stocks
Vero cells (ATCC) were maintained with Minimal Essential Media (MEM, Cellgro) supplemented with 100 IU penicillin/ml, 100 μg streptomycin/ml, 2.5 μg amphotericin B/ml, and 10% heatinactivated fetal bovine serum (HI-FBS, Hyclone). Cells were incubated at 37°C, 5% CO2 in a humidified chamber. L, Gro2C, and sog9 cell lines were maintained with Dulbecco’s Modified Essential Media (D-MEM) supplemented with 1 μg/ml ceftriaxone and 10% HI-FBS.
HSV1 KOS (a kind gift from David Bloom, Univ. of Florida College of Medicine), HSV1-1(KOS)ΔgC2-3 (a kind gift from Robert Visalli, Indiana University Purdue University), GHSV-UL46 (ATCC) expressing GFP linked to the tegument protein, VP11/12, HSV2 (ATCC), and EHV (ATCC) were propagated in Vero cells. At full CPE, cells were pelleted, resuspended in MEM, 2% FBS, and freezethawed three times followed by sonication for 2 minutes. Cell debris was removed by centrifugation, and viral stocks were aliquoted and stored at -80°C. For some experiments, HSV1 KOS and GHSV were purified using an Iodixanol step gradient by centrifugation at 140,000xg for 3 hours in an SW28 followed by removal of the virus band located at the 20% to 30% interface. The virus band was diluted in MEM, 2% FBS and pelleted through a 20% Iodixanol pad. The virus pellet was then resuspended in MEM, 2% FBS, and stored at -80°C. Virus stocks were titered on Vero cells in the presence of 0.3% human gamma globulin (Sigma), and plaques were visualized three days later by staining with 0.1% crystal violet in 20% ethanol.
Extracts
Melissa officinalis extract was obtained from Vital Force Naturopathic Compounding (Phoenix, AZ). Briefly, verified and dried Melissa officinalis leaves were ground to a fine powder. The powder was resuspended at a 1:8 ratio (solid: 75% glycerin) and incubated for 2 days at room temperature. The bulk botanical material then was removed by centrifugation at 3,000xg for 15 minutes and the supernatant filtered through a 0.2 μm filter. The final extract was stored at room temperature. For reference and standardization, all extractions were consistently found to contain concentrations of non-volatile solutes ranging between 25-30 mg/ml extract.
Cell viability
Vero cells were incubated with increasing concentrations of Melissa officinalis extract or vehicle for 24 hours. Cells were harvested by incubation with 0.25% trypsin for 5 minutes followed by gentle resuspension. Cells were diluted in PBS and mixed 1:1 with 0.4% trypan blue (Invitrogen). Percent viability was determined by counting clear versus blue cells using an Invitrogen Countess.
Plaque reduction assays and single cycle growth curves
Plaque reduction assays were performed by diluting virus stocks and preincubating 100-200 plaque-forming units (pfu) with increasing concentrations of Melissa officinalis extract or vehicle (75% glycerol) for 20 minutes. Monolayers were infected for 1 hour at 37°C followed by incubation in media containing Melissa officinalis or vehicle for 3 days at 37°C. Plaques were visualized by staining with 0.1% crystal violet in 20% ethanol.
Single cycle growth curves were performed by infecting Vero cells in duplicate with a multiplicity of infection (MOI) of 5 with HSV1 KOS. Increasing concentrations of Melissa officinalis or vehicle were incubated with virus for 20 minutes followed by infection for 1 hour at 37°C. Infected cells were washed twice with warm PBS, given fresh media containing Melissa officinalis or vehicle, and were harvested at 12 hour intervals. Infected cells plus media were then pelleted at 12,000 × g for 30 minutes, resuspended in MEM, 2% FBS, and stored at -80°C. Samples were freeze-thawed three times, sonicated for 2 minutes, and titered by plaque assay.
Binding/Uptake assays
Binding of HSV1 to Vero cells was assayed by adding 100-200 pfu HSV1 KOS to increasing concentrations of Melissa officinalis or vehicle 20 minutes prior to infection. Virus was added to prechilled Vero cell monolayers and incubated for 2 hours at 4°C to allow binding. Cells were washed two times with PBS to remove unbound virus, complete media added, and the cells incubated for 3 days at 37oC followed by crystal violet staining to visualize plaque formation.
Uptake of HSV1 into Vero cells was assayed by infecting prechilled Vero cell monolayers with 100-200 pfu HSV1 KOS for 2 hours at 4°C in complete media (without Melissa officinalis) to allow for cell binding. Monolayers were washed with PBS to remove unbound virus, media was then added which contained increasing concentrations of Melissa officinalis or vehicle, and incubated at 37°C for 3 days followed by crystal violet staining to visualize plaque formation.
Western blots
HSV1 KOS was used to infect Vero cells at an MOI of 5. Melissa officinalis was added to virus at various times prior to infection, at the time of infection, or at various times following infection. Monolayers were fed with 3 mls of complete media or media containing Melissa officinalis or vehicle after 1 hour of incubation at 37°C. Following 18 hours of incubation, cells were washed two times with PBS and lysed with 1X SDS sample buffer (125mM Tris-Cl, pH 6.8, 25% glycerol, 2.5% SDS, 100mM β-mercaptoethanol, 0.025% bromophenol blue, 10% Protease inhibitor cocktail (Thermo Scientific)). Samples were separated on 10% polyacrylamide gels, transferred to PVDF membrane in blotting transfer buffer (10mM CAPS, 20% methanol), and blocked in blotto (25mM Tris, pH 7.5, 137mM NaCl, 2.5 mM KCl, 0.025% Tween, 3% powdered milk). Mouse monoclonal antibodies to ICP4 (a kind gift from David Bloom, Univ. of Florida College of Medicine), ICP8, gC, VP16, GAPDH (Abcam), and gD (Virusys) were diluted according to manufacturer’s specifications. Detection was performed using secondary goat anti-mouse IgG or goat anti-rabbit IgG conjugated to horseradish peroxidase (Santa Cruz) in the presence of a chemiluminescent substrate (Biorad).
Immunofluorescence
Iodixanol-purified GHSV-UL46 was used to infect Vero cells seeded at 25% confluency overnight on a glass cover slip at an MOI of 50. Various concentrations of Melissa officinalis or vehicle were added to virus 20 min prior to infection, and cells were incubated at 4°C for 2 hours to allow virus binding. Cells were washed with PBS, fixed in 4% formaldehyde (Thermo Scientific), and quenched in 50mM NH4Cl. Cell membrane was stained with 5 μg/ ml Wheat Germ Agglutinin (WGA, Molecular Probes) and nuclei were stained with 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI, Sigma). Cover slips were mounted onto glass slides with Prolong Antifade Reagent (Life Technologies) and visualized with a Leica TCS SP8 Confocal Laser Scanning Microscope using a 40X lens (performed at Veteran’s Administration Hospital Research Center, Phoenix, Arizona).
Heparin-agarose binding assays
Heparin conjugated to agarose (Sigma) was incubated with 1 × 106 pfu HSV1 KOS in the absence of competitor or in the presence of various concentrations of Melissa officinalis extract for 1 hour at 4°C. Heparin-agarose was washed and pelleted two times followed by the addition of 1 × SDS sample buffer. Samples were analyzed for HSV1 binding by Western blot for the presence of HSV1 gB. Control samples included non-conjugated agarose alone and the addition of vehicle in place of Melissa officinalis (data not shown).
Virion stability assay
A 20% sucrose pad was loaded with 1 × 106 pfu HSV1 KOS that had been untreated or treated with increasing concentrations of Melissa officinalis, vehicle, or 0.5% Triton ×-100 for 1 hour at 37°C. Samples were centrifuged at 140,000xg for 80 minutes in an SW55 rotor, and pellets were resuspended in 1 × SDS sample buffer followed by Western blot analysis for tegument protein, VP16, and outer membrane protein, gD.
Depletion assay
His-tagged HSV1 glycoproteins from the virulent McKrae strain were expressed from plasmids, pPEP98 (gB) and pPEP99 (gD) (a kind gift from Vladimir Chouljenko, Louisiana State University). Vero cells were transfected with each plasmid using Lipofectamine 2000. Forty-eight hours post-transfection, cells were lysed in NP-40 lysis buffer. The his-tagged glycoproteins B and D were subsequently purified using an anti-6X His antibody conjugated agarose resin. The binding of the gB and gD to the resin was verified by Western blot analysis. Melissa officinalis extract (100 μl) was incubated with the resin alone or with resin bound with glycoprotein B or D. Following 30 min incubation at 4°C, the resin was pelleted at low speed, and the supernatant removed and assayed for HSV1 inhibitory activity in a plaque assay.
Statistics
Statistical analysis was performed using Prism 6 for MAC OS X, GraphPad Software, Inc. For immunofluorescence, a one way ANOVA followed by a Turkey test for multiple comparisons was performed to determine differences between groups.
Results
Extracts of Melissa officinalis inhibit HSV1 cytopathic effect and plaque formation
Extracts from the plant, Melissa officinalis, have been reported to affect replication of HSV1 while having low toxicity to cell monolayers [23,34]. These results were confirmed by incubating increasing concentrations of Melissa officinalis extract with Vero cell monolayers alone and by incubating increasing concentrations of Melissa officinalis or vehicle with Vero cells infected with HSV1. Figure 1A shows that concentrations of Melissa officinalis up to 18 μl/ml of extract had no microscopically visible effects on growth or morphology of the Vero cells. When cells were infected with HSV1, typical cytopathic effect (CPE) due to viral infection was seen in untreated cells (Figure 1B). Upon the addition of Melissa officinalis during HSV1 infection, CPE was still observed with 0.1 μl/ml extract, but was greatly reduced at 0.3 μl/ml, and completely inhibited at 1-3 μl/ml extract. The same concentrations of vehicle did not inhibit CPE due to HSV1 infection (Figure 1B).
Figure 1: Melissa officinalis inhibits HSV1-induced CPE. (A) Vero cell monolayers were treated with increasing concentrations of Melissa officinalis and photographed 24 hours later. (B) Vero cell monolayers were infected with HSV1 at a MOI=5, treated with increasing concentrations of Melissa officinalis or vehicle, and photographed at 24 hpi.
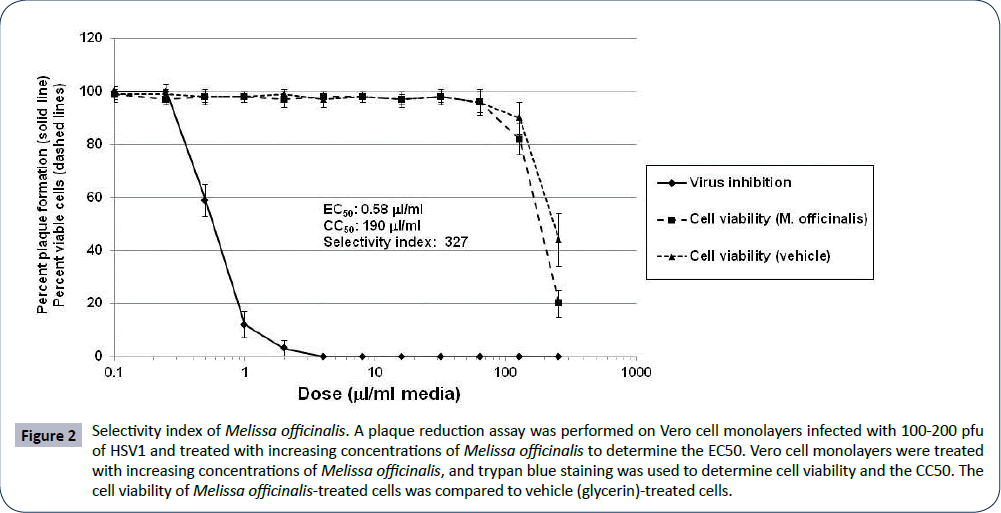
The selectivity index (SI) for Melissa officinalis extract was calculated by incubating HSV1-infected Vero cells with increasing concentrations of Melissa officinalis and by incubating Vero cells with increasing concentrations of Melissa officinalis alone. The EC50, or effective concentration at which 50% of HSV1 plaque formation was inhibited by Melissa officinalis, was determined to be 0.58 μl/ml extract (Figure 2). The CC50, or cytotoxic concentration at which 50% of the cells were nonviable following treatment with Melissa officinalis was 190 μl/ml (Figure 2). This gave an SI of Melissa officinalis extract (CC50/EC50) of 327. Notably, the cell viability curve and CC50 with vehicle alone (75% glycerin) was very similar to that following treatment with Melissa officinalis (Figure 2). This suggests that the cell toxicity of the Melissa officinalis extract was likely associated with the extraction solution (75% glycerol) and that the SI of Melissa officinalis may be higher than 327.
Figure 2: Selectivity index of Melissa officinalis. A plaque reduction assay was performed on Vero cell monolayers infected with 100-200 pfu of HSV1 and treated with increasing concentrations of Melissa officinalis to determine the EC50. Vero cell monolayers were treated with increasing concentrations of Melissa officinalis, and trypan blue staining was used to determine cell viability and the CC50. The cell viability of Melissa officinalis-treated cells was compared to vehicle (glycerin)-treated cells.
Melissa officinalis decreases infective titers of HSV1 in a single cycle growth assay
Vero cells were infected with HSV1 and treated with 0.3 or 1 μl/ml Melissa officinalis or vehicle. At 1, 12 and 24 hours post infection, virus was harvested and titered. Figure 3 shows that the titer of untreated HSV1 increased by nearly 3-logs at 24 hours post infection (hpi) as compared to the input virus titer at 1 hpi. HSV1 treated with either 0.3 or 1 μl/ml vehicle had a similar pattern of growth in cells as untreated virus. The addition of Melissa officinalis at 0.3 and 1 μl/ml prior to HSV1 infection resulted in a rapid 1- and 2-log decrease in titer, respectively, as measured at 1 hpi. Melissa officinalis treatment at 0.3 μλ/ml resulted in no additional change in titer at 12 hpi, but by 24 hpi, viral titers increased by 3-logs to a level approximately 1-log less than untreated virus (Figure 3). Melissa officinalis treatment at 1 μl/ml resulted in a 1.5-log decrease in measurable titers at 12 hpi, but by 24 hpi a 2.5-log increase in virus titer as compared to input titer was measured. This final titer at 24 hpi was approximately 2-logs less than untreated virus (Figure 3). Therefore, initial treatment with Melissa officinalis resulted in a rapid decrease in infective titers, but all infections were able to partially recover by 24 hpi.
Melissa officinalis inhibited HSV1 when added prior to infection
Based on the results from Figure 2, Melissa officinalis appeared to likely inhibit HSV1 during the initial steps of infection. To further evaluate this, Melissa officinalis was incubated with HSV1 for various times prior to infection, at the same time of infection, or at various times post infection followed by analysis of viral protein expression. HSV1 treated with 1 μl/ml Melissa officinalis was compared to untreated or vehicle-treated HSV1 by measuring the accumulation of immediate early (IE), early (E), and late (L) viral proteins. Figure 4A shows that untreated and vehicle-treated HSV1 resulted in similar expression of ICP4 (IE), ICP8 (E), and gC (L) proteins at 18 hpi. Alternately, HSV1 virions treated with Melissa officinalis for 60 or 30 minutes prior to infection or added at the same time of infection resulted in complete loss of viral protein expression (Figure 4A). Treatment of HSV1-infected cells 30 minutes post-infection resulted in a slight decrease in protein expression for all viral proteins whereas treatment 60 or 120 minutes post infection did not significantly reduce the levels of IE, E, and L proteins (Figure 4A).
Figure 4: Melissa officinalis inhibits HSV1 during pretreatment or at the time of infection. (A) HSV1 was left untreated, treated with vehicle, or treated with 1 μl/ml Melissa officinalis at various times prior to infection, at the time of infection, or at various times post infection. At 18 hpi cells were harvested, and Western blot analysis was performed to detect IE (ICP4), E (ICP8), and L (gC) viral proteins. All samples were normalized to GAPDH. (B) HSV1 was left untreated or was treated with 1 μl/ml Melissa officinalis 30 min prior to infection, at the time of infection, or at various time post infection. At 24 hpi monolayers were harvested and titered for plaque forming units. Input virus (at 1 hpi) had a titer of 5 × 103 pfu/ml (not shown).
A similar experiment was performed that measured viral replication following incubation with Melissa officinalis. Again, 1 μl/ml Melissa officinalis was incubated with HSV1 for 30 minutes prior to infection, at the same time of infection, or at various times post infection and analyzed for the production of infectious virions at 24 hpi. At 1 hpi the amount of input virus was measured, and at 24 hpi a 4.5-log increase in titer was observed in untreated samples (Figure 4B). However, when virus was pretreated with Melissa officinalis for 30 min or added at the same time as infection, no HSV1 was detected at 24 hpi. When Melissa officinalis was added 30 min post infection, a 1-log decrease in yield as compared to input titer was observed. This agrees with results seen with protein expression which shows that EI, E, and L protein production is decreased as compared to untreated virus. Similarly, the addition of Melissa officinalis at 1, 2, 4, and 6 hours post infection resulted in approximately 3-, 2-, 1-, and 1-log reductions in yield, respectively.
Melissa officinalis at low concentrations inhibits HSV1 virions from binding to the cell
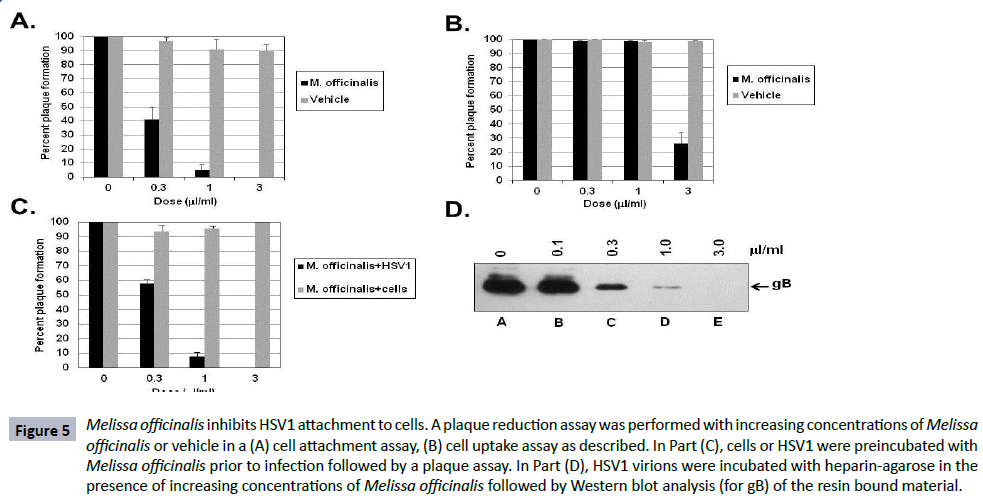
The results of treatment of HSV1 in the previous single cycle growth and protein synthesis assays suggest that Melissa officinalis has an effect prior to or during binding of the virion to the cell. A virus binding assay was performed by adding increasing concentrations of Melissa officinalis to HSV1, incubating the virus with cells at 4oC to allow for adsorption, followed by washing away unbound virus and botanical extract, and then incubating at 37°C to allow any bound virus to replicate and form plaques. Figure 5A shows that a concentration of 0.3 μl/ml and 1 μl/ml was able to inhibit 60% and 95% of the virus from binding to cells, respectively, whereas concentrations of vehicle up to 3 μl/ml had no effect on binding and plaque formation.
Figure 5: Melissa officinalis inhibits HSV1 attachment to cells. A plaque reduction assay was performed with increasing concentrations of Melissa officinalis or vehicle in a (A) cell attachment assay, (B) cell uptake assay as described. In Part (C), cells or HSV1 were preincubated with Melissa officinalis prior to infection followed by a plaque assay. In Part (D), HSV1 virions were incubated with heparin-agarose in the presence of increasing concentrations of Melissa officinalis followed by Western blot analysis (for gB) of the resin bound material.
In addition, a virus uptake assay was performed by incubating HSV1 with cells at 4°C to initially allow virus to bind to the cell followed by the addition of media containing increasing concentrations of Melissa officinalis or vehicle with incubation at 37°C. Figure 5B shows that 0.3 μl/ml and 1 μl/ml had no effect on uptake of the virus, whereas a higher concentration of 3 μl/ml inhibited uptake of 70% of the virus into the cells. Vehicle had no effect on uptake and plaque formation.
Next, assays were done to test if the effect of Melissa officinalis was specific for the virus and or the cells. Figure 5C shows that when cells were pre-incubated with increasing concentrations of Melissa officinalis followed by washing and then infecting with HSV1, concentrations up to 3 μl/ml did not affect HSV1 infectivity or replication. However, when various concentrations of Melissa officinalis were pre-incubated with HSV1 virions followed by pelleting of the virus, resuspending in fresh media, and infecting cells, a dose dependent response was observed where 0.3 and 1 μl/ml inhibited plaque formation by 42% and 92%, respectively.
These results together suggest that Melissa officinalis components may be interacting with the free HSV1 virion and inhibiting viral attachment to the cell. HSV1 has previously been shown to bind to heparin in vitro as a surrogate molecule to the heparan sulfate moieties present on the cell surface [47]. As an in vitro assay, HSV1 virions (1 × 106 pfu) were incubated with heparin-agarose either alone or with increasing concentrations of Melissa officinalis. The agarose beads were washed and bound virus eluted by boiling in SDS-sample buffer. Western blot analysis of the eluted material (testing for HSV1 gB) showed that 0.3, 1, and 3 μl/ml was able to effectively reduced the amount of gB present on the resin by 79%, 96% and 100%, respectively (Figure 5D). HSV1 did not bind to agarose alone and incubation with vehicle had no effect on virion binding to the heparin-agarose (data not shown).
The inhibition of virion binding to the cell by Melissa officinalis was confirmed by performing a binding assay using HSV1 expressing a GFP-labeled tegument protein (GHSV). Figure 6A shows full color images and gray-scale images of GFP for each field (representative image from z-stack). In mock-infected Vero cells, only the cell membrane (stained with WGA) and nucleus (stained with DAPI) could be observed (Figure 6A, panels A and E). In cells infected with GHSV, GFP-fluorescent virions binding to the surface of the cells were clearly observed (Figure 6A, panels B and F). When cells were infected with GHSV in the presence of 3 μl/ml vehicle, a slight reduction in virion particles bound to the cell was observed, however this did not show statistical significance (Figure 6A, panels C and G and Figure 6B). When cells were infected with GHSV in the presence of 0.3 μl/ml Melissa officinalis, an 88% reduction in the number of viral particles bound per cell was observed (Figure 6A, panels D and H and Figure 6B), while 3 μl/ml Melissa officinalis resulted in a complete loss of virion binding (Figure 6B).
Figure 6: Melissa officinalis inhibits GFP-labeled HSV1 from binding to cells. (A) Confocal microscopy. A binding assay was performed with GHSV at an MOI=50 followed by staining with WGA-Alexa Fluor® 594 and DAPI. Panels A, B, C, and D are full color, and panels E, F, G, and H are gray-scale panels of GFP only. Treatment conditions are listed above the panels. (B) Quantitative analysis of virion binding. Three fields from Part (A) were analyzed with 10 cells per sample and quantitated for the number of bound virions. * indicates p<0.0001.
Melissa officinalis disrupts virion structure only at high concentrations
It is possible that Melissa officinalis reduces virus binding to the cell by either blocking virus interaction with cell receptors or by disrupting virion structure. To test for virus stability, HSV1 virions were treated with increasing concentrations of Melissa officinalis or vehicle followed by pelleting through a sucrose pad. Concentrations of vehicle up to 3 μl/ml did not affect stability of the virions as seen by the presence of the envelope gD and internal tegument protein (VP16) in the sucrose pellet (Figure 7). Concentrations of Melissa officinalis up to 1 μl/ml also did not affect virion stability; however Melissa officinalis at 3 μl/ml caused disruption of the virions resulting in loss of envelope and tegument proteins (Figure 7). Treatment with Triton X-100 was used as a control to show complete disruption of virions. These results may suggest that Melissa officinalis may bind to the virus and block cell attachment at low concentrations (1 μl/ml or less), whereas higher concentrations (3 μl/ml or more) may affect virion stability.
Figure 7: High dose Melissa officinalis disrupts virion structure. HSV1 virions were left untreated, treated with 0.5% Triton, or were treated with increasing concentrations of Melissa officinalis or vehicle for 1 hour at 37°C. Virions were pelleted through a 20% sucrose pad, resuspended in 1X SDS sample buffer, and analyzed by Western blot for envelope (gD) and tegument proteins (VP16).
Glycoprotein B binds to the antiviral component(s) present in Melissa officinalis
The HSV1 glycoproteins are involved in binding and uptake into the cell. Initial binding involves gB and gC and therefore represent likely targets for the antiviral activity associated with Melissa officinalis. In order to test the role of gC, an HSV1 deleted for the gC gene (HSV1-1(KOS)ΔgC2-3) was used to test for the ability of Melissa officinalis to inhibit viral replication. HSV1 deleted for gC is a weak but viable virus [36,37]. As shown in Figure 8A, Melissa officinalis gave similar inhibition curves for wild type and HSV1-1(KOS)ΔgC2-3 with 1 μl/ml inhibiting plaque formation by 90% in both cases. This suggests that a component of extract from Melissa officinalis does not bind to gC, but rather may be interacting with gB on the virion.
Figure 8: A component(s) of Melissa officinalis binds to gB to inhibit HSV1. (A) Vero cells were infected with 100-200 pfu HSV1 or HSV1 deleted for the gC gene (HSV1-1(KOS)ΔgC2-3) and treated with increasing concentrations of Melissa officinalis or vehicle. (B) His-tagged HSV1 glycoproteins, gB and gD, were expressed in Vero cells and purified using an anti-His – agarose resin. Melissa officinalis extract was incubated with anti-His – agarose resin alone or with resin bound with gB or gD. The resin was pelleted and the supernatant assayed for virus inhibition using a plaque reduction assay.
A gB deficient HSV1 is not viable, so to examine the role of gB, histagged HSV1 gB and gD were transiently expressed in Vero cells and purified by binding to a 6X his-tag antibody-agarose resin. Melissa officinalis extract was incubated with resin alone or with resin containing bound gB or gD, and the resulting supernatant (unbound extract components) were removed and tested for the ability to inhibit HSV1 plaque formation. Figure 8B shows that both resin-bound Melissa officinalis extract and extract incubated with gD-resin exhibited similar inhibition of plaque formation. However, extract incubated with gB-resin lost approximately 30- 40% of the HSV1 inhibitory activity. These results suggest that the HSV1 inhibitory component present in Melissa officinalis could be depleted by binding to gB. Cumulatively, these results support that Melissa officinalis inhibits HSV1 attachment to the cell by binding to and blocking gB receptor interaction with the cell.
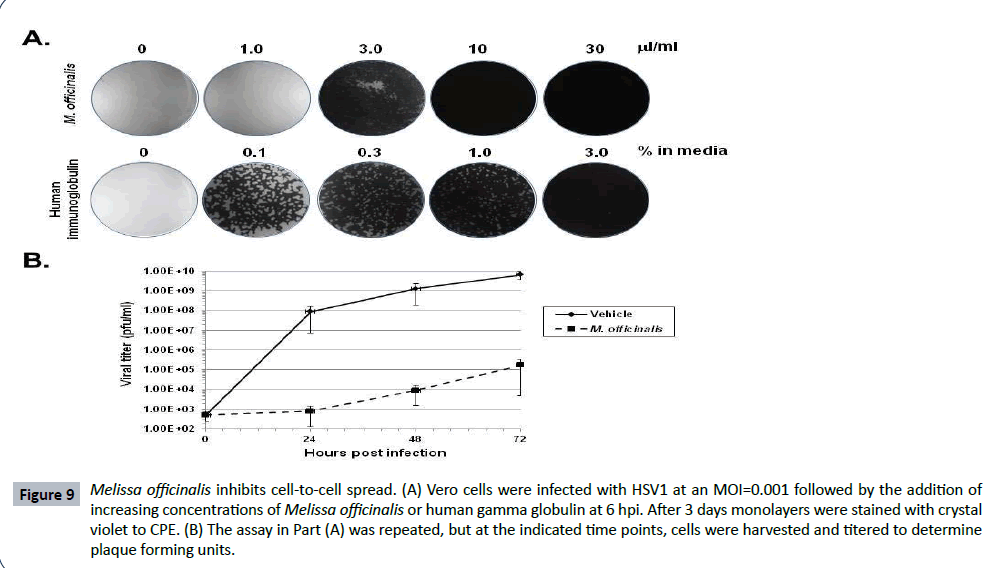
Higher concentrations of Melissa officinalis can inhibit cell to cell spread
HSV1 gB plays an essential role not only for viral attachment, but for cell-to-cell spread as well [48]. To test for the ability of Melissa officinalis to inhibit viral spread, Vero cell monolayers were infected with HSV1 at an MOI=0.001, and at 6 hpi following binding and uptake, the cells were washed followed by the addition of increasing concentrations of Melissa officinalis or human gamma-globulin. Infections were allowed to proceed for 3 days followed by staining of the monolayers. Although 1 μl/ml Melissa officinalis did not prevent spread and destruction of the monolayer, a concentration of 3 μl/ml resulted in only limited CPE of the cell monolayer (Figure 9A). At concentrations of 10 and 30 μl/ml, no viral spread of CPE was observed (Figure 9A). These results suggest that Melissa officinalis can inhibit both HSV1 attachment and cell-to-cell spread, although a higher concentration is required for the inhibition of cell-to-cell spread. As a control, this assay was done in comparison to treatment with human gamma-globulin. Human gamma-globulin binds free virions on the Fc receptor which is formed from a complex of glycoprotein E and glycoprotein [49]. A dose dependent effect of human gamma globulin was observed where doses from 0.1- 3.0% produced a gradient of reduced CPE. This is a very different result than that observed with Melissa officinalis where a gradient effect was not observed with full CPE occurred at 1 μl/ml, while at 3 μl/ml the monolayer had only minimal CPE. To quantify this effect, Figure 9B shows titers measured from infected cell monolayers treated with 3 μl/ml Melissa officinalis or vehicle. At all-time points measured over the course of 3 days, Melissa officinalis inhibited viral replication by 4-5 logs as compared to vehicle.
Figure 9: Melissa officinalis inhibits cell-to-cell spread. (A) Vero cells were infected with HSV1 at an MOI=0.001 followed by the addition of increasing concentrations of Melissa officinalis or human gamma globulin at 6 hpi. After 3 days monolayers were stained with crystal violet to CPE. (B) The assay in Part (A) was repeated, but at the indicated time points, cells were harvested and titered to determine plaque forming units.
Melissa officinalis inhibits the replication of other viruses
The ability of Melissa officinalis to inhibit other related members of the alpha herpes virus family was tested by plaquing HSV1, HSV2, and equine herpes virus 1 (EHV1) on Vero cell monolayers with increasing concentrations of extract from Melissa officinalis. HSV2 was slightly more sensitive to Melissa officinalis as compared to HSV1 (Figure 10A and B), whereas EHV1 was intermediate in sensitivity. In Figure 10A, the inhibition of viral induced CPE could easily be observed in HSV1 infected cells at 6 μl/ml, HSV2 at 2 μl/ml, and EHV1 at 18 μl/ml. To quantitate this inhibition more accurately, the plaque reduction assay, shown in Figure 10B top panel, demonstrates the ID50 for HSV1 at 0.8 μl/ml, HSV2 at 0.2 μl/ml, and EHV1 at 6.0 μl/ml.
Figure 10: Melissa officinalis inhibits alpha herpes viruses and members of other virus families. (A) Vero cell monolayers were infected with 100-200 pfu HSV1, HSV2, and EHV1 with increasing concentrations of Melissa officinalis. After 3 days of incubation plaques were photographed. (B) Appropriate cell monolayers were infected with 100-200 pfu of virus in the presence of increasing concentrations of Melissa officinalis. After incubation, plaques were stained with crystal violet for quantitation. Left panel - HSV1 (•), HSV2 (ϒ), and EHV ( ). Right panel - HSV1 (•), VSV (ϒ), EMCV (ρ), VACV (λ), ADENO ( ), REO (ο), and SV40 (|).
A number of additional virus families were tested for their sensitivity to Melissa officinalis using various prototype viruses. Vaccinia virus (VACV-Copenhagen, Orthopoxviridae) had similar sensitivity as HSV-1 to increasing concentrations of Melissa officinalis, whereas vesicular stomatitis virus (VSV, Rhabdoviridae), adenovirus (ADENO, Adenoviridae), and simian virus 40 (SV40, Papovaviridae) had intermediate sensitivity to Melissa officinalis with ID50 ranging from 3.0-7.5 μl/ml (Figure 10B). Structurally, these intermediately sensitive viruses are both enveloped and non-enveloped. Encephalomyocarditis virus (EMCV, Picornaviridae) and reovirus (REO, Reoviridae) were unaffected by the presence of Melissa officinalis during infection (Figure 10B).
Discussion
This study sought to further define the antiviral activity associated with Melissa officinalis. As shown, Melissa officinalis strongly inhibited HSV1-induced CPE and plaque formation. The extract was relatively nontoxic to cells with a high SI of 327, thereby supporting its potential for use as a therapeutic. Astani et al. and Nolkemper et al. have reported SIs of 875 and 2200, respectively, for extracts of Melissa officinalis. Both groups resuspended their dried material in boiling water rather than glycerin which was used in our current study [23,34]. These previous results agree with our results since cell toxicity with glycerin alone was nearly identical as the glycerin-extracted Melissa officinalis.
HSV1 virions were shown to be stable up to 1 μl/ml following incubation with extract from Melissa officinalis, however at 3 μl/ ml, the virions become disrupted as evidenced by loss of both envelope and tegument proteins. Therefore it appears that two phenomena may be occurring with the extract from Melissa officinalis: inhibition of HSV1 binding at low concentrations (≤ 1 μl/ml), and disruption of virion structure at ≥ 3 μl/ml. These two phenomena are evidenced in the concentrations of Melissa officinalis required to inhibit HSV1 in the binding and uptake assays. In the binding assay HSV1 was preincubated with Melissa officinalis followed by infection of cell monolayers with the mixture at 4°C. A concentration of 0.3 μl/ml Melissa officinalis resulted in a 60% reduction in plaque formation and at 1 μl/ml, a 90% reduction. At these concentrations of extract, the HSV1 virions were stable, so the effect of Melissa officinalis was to directly inhibit binding of virions to the cell. The ability of Melissa officinalis to act directly on virions to prevent binding to the cell has also been suggested by Nolkemper et al. and Astani et al. [23,34]. Alternately, in the uptake assay, HSV1 was prebound to cells at 4°C followed by the addition of Melissa officinalis. Doses below 3 μl/ml did not affect viral plaque formation, but a concentration of 3 μl/ml was able to inhibit 74% of plaque formation. This result may suggest that following virus binding, a higher concentration of Melissa officinalis may lead to disruption of the virus particles already bound to the cell. Therefore, Melissa officinalis inhibits virion binding to the cell at lower concentrations, but inhibition of uptake requires a concentration that disrupts virion structure.
The direct inhibition of HSV1 virions by Melissa officinalis is also supported by the data in the preincubation assay (Figure 5C). When increasing concentrations of Melissa officinalis were preincubated with cell monolayers followed by infection with HSV1, no significant reduction in plaque formation was observed. However, when HSV1 virions were preincubated with Melissa officinalis followed by infection of cell monolayers, 0.3 μl/ml inhibited plaque formation by 42%. This supports and suggests that components in Melissa officinalis bind directly to the virus and prevent attachment to the cell. In addition, immunofluorescence data showed that 0.3 μl/ml inhibited nearly 80% of virions from binding to the cells.
The initial step of HSV1 in binding to a cell occurs when virusencoded glycoproteins, gB or gC, bind to heparan sulfate moieties [36,37]. It has been found that soluble heparin, which is related in structure, can be used to inhibit HSV1 binding to the cell [47,50]. In addition, we found that extracts from Melissa officinalis could inhibit HSV1 binding to heparin-agarose in vitro. This inhibition occurred at the lower concentrations at which virion structure remained intact (0.3 and 1 μl/ml).
As mentioned, the HSV1 gB and gC are involved in initial cell attachment. WtHSV1 and HSV1 deleted of gC were similarly affected by Melissa officinalis extract suggesting the gC at least is not the sole component targeted the active botanical constituent. More importantly, resin-bound gB was able to partially deplete the inhibitory compound(s) from the Melissa officinalis extract. This key experiment supports our previous results and suggest that the active compound(s) present in Melissa officinalis binds directly to gB to inhibit virus binding to cells. Density gradient analysis of virions incubated with 1 μl/ml Melissa officinalis did not cause an increase in density due to components of Melissa officinalis binding to virions as seen previously with HIV (data not shown) [51]. This lack of shift in density may be due to the relatively low molar amounts of gB present on HSV1 virions [52].
Murine L-cell fibroblast cell lines that are deficient in heparan sulfate (Gro2C) or are deficient in both heparan sulfate and chondroitin sulfate (sog9) have previously been reported [53,54]. When these cells were tested under single cycle conditions, a concentration of 1 μl/ml Melissa officinalis inhibited HSV1 yields by 90% in each cell line tested, regardless of the presence of heparan sulfate and/or chondroitin sulfate (data not shown). This is in agreement with data from Bender et al. that shows gB can bind cells independently of heparan sulfate by using alternative receptors [54-58]. This indirectly supports that the inhibitory component of Melissa officinalis likely binds to gB thereby prevents interactions with either heparan sulfate or other structurally related cellular receptors.
When comparing other alpha herpes viruses for their sensitivity to Melissa officinalis, HSV2 appeared to be the most sensitive, followed closely by HSV1, and EHV1 being more intermediate. All three viruses utilize heparin sulfate for cell attachment. The reduced activity against EHV1 was surprising, but may be related to structural differences in the viral gB. For HSV1, Melissa officinalis also inhibited cell-to-cell spread of the virus. The requirements for virion binding/entry and cell-cell fusion are not identical, however gD, gHgL and gB are essential for both processes [48]. The inhibitory activity of Melissa officinalis targeting gB agrees with its effect on both viral attachment and cell-to-cell spread. Higher doses of Melissa officinalis were required to inhibit cell-tocell spread, but this may be related to gB mechanisms of action or accessibility during viral attachment versus cell-cell fusion.
When a variety of other viral families were tested for the ability of Melissa officinalis extract to inhibit replication, strong inhibitory activity towards VACV was observed. VACV infection can be inhibited by soluble heparan sulfate, in agreement with the ability of Melissa officinalis to inhibit infection [59]. VSV, adenovirus and SV40 were moderately inhibited by Melissa officinalis. VSV has not been shown to bind to heparan sulfate, however it requires a charged interaction for binding to the cell, and negatively charged molecules have been shown to inhibit infection possibly explaining this partial inhibition [60]. Adenovirus has been shown to bind heparan sulfate as well as various other cell surface molecules [61,62]. This broad range of cell surface receptors may support the partial inhibition observed. For SV40, viral binding involves the cellular glycolipid, GM1, which contains a single sialic acid moiety [63]. It is possible again that Melissa officinalis partially inhibits interaction of the virus particle with this receptor. The final two viruses in our study that were not inhibited by Melissa officinalis, EMCV and Reovirus, both bind to sialic acid on the surface of the cell [64-66]. The results suggest that Melissa officinalis was not able to bind to these viruses and inhibit cell attachment. Together, the viral family results support that component(s) of Melissa officinalis are able to bind viral envelope or capsid proteins and prevent interaction with cellular heparan sulfate or related receptors.
Acknowledgements
The authors would like to thank Gary H. Cohen and Roselyn J Eisenberg for their generous gift of L, Gro2C, and sog9, Dr. Vladimir Chouljenko for the plasmids, pPEP98 (gB) and pPEP99 (gD), David Bloom for HSV1 KOS and for antibodies to ICP4, and the Veteran’s Administration Hospital Research Center (Phoenix, Arizona) for use of their confocal microscopy facility.
References
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, et al. (2006) Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 296: 964-973.
- Smith JS, Robinson NJ (2002) Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis 186 Suppl 1: S3-28.
- Chayavichitsilp P, Buckwalter JV, Krakowski AC, Friedlander SF (2009) Herpes simplex. Pediatr Rev 30: 119-129.
- Arduino PG, Porter SR (2008) Herpes Simplex Virus Type 1 infection: overview on relevant clinico-pathological features. J Oral Pathol Med 37: 107-121.
- Fraser NW, Lawrence WC, Wroblewska Z, Gilden DH, Koprowski H (1981) Herpes simplex type 1 DNA in human brain tissue. ProcNatlAcadSci USA 78: 6461-6465.
- Gerdes JC, Smith DS, Forstot SL (1981) Restriction endonuclease cleavage of DNA obtained from herpes simplex isolates of two patients with bilateral herpetic disease. Curr Eye Res 1: 357-360.
- Warren KG, Brown SM, Wroblewska Z, Gilden D, Koprowski H, et al. (1978) Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med 298: 1068-1069.
- Warren KG, Koprowski H, Lonsdale DM, Brown SM, Subak-Sharpe JH (1979) The polypeptide and the DNA restriction enzyme profiles of spontaneous isolates of herpes simplex virus type 1 from explants of human trigeminal, superior cervical and vagus ganglia. J Gen Virol 43: 151-171.
- Usatine RP, Tinitigan R (2010) Nongenital herpes simplex virus. AmFam Physician 82: 1075-1082.
- Garner JA (2003) Herpes simplex virion entry into and intracellular transport within mammalian cells. Adv Drug Deliv Rev 55: 1497-1513.
- Perng GC, Jones C (2010) Towards an understanding of the herpes simplex virus type 1 latency-reactivation cycle. InterdiscipPerspect Infect Dis 2010: 262415.
- Reardon JE, Spector T (1989) Herpes simplex virus type 1 DNA polymerase. Mechanism of inhibition by acyclovir triphosphate. J Biol Chem 264: 7405-7411.
- Kost RG, Hill EL, Tigges M, Straus SE (1993) Brief report: recurrent acyclovir-resistant genital herpes in an immunocompetent patient. N Engl J Med 329: 1777-1782.
- Kimberlin DW, Crumpacker CS, Straus SE, Biron KK, Drew WL, et al. (1995) Antiviral resistance in clinical practice. Antiviral Res 26: 423-438.
- Wagstaff AJ, Bryson HM (1994) Foscarnet. A reappraisal of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with viral infections. Drugs 48: 199-226.
- Ho DY (1992) Herpes simplex virus latency: molecular aspects. Prog Med Virol 39: 76-115.
- Safrin S, Cherrington J, Jaffe HS (1999) Cidofovir. Review of current and potential clinical uses. Adv Exp Med Biol 458: 111-120.
- Noormohamed FH, Youle MS, Higgs CJ, Martin-Munley S, Gazzard BG, et al. (1998) Pharmacokinetics and absolute bioavailability of oral foscarnet in human immunodeficiency virus-seropositive patients. Antimicrob Agents Chemother 42: 293-297.
- Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, et al. (1998) Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA 280: 1569-1575.
- Kessler RC, Davis RB, Foster DF, Van Rompay MI, Walters EE, et al. (2001) Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann Intern Med 135: 262-268.
- Okoro CA, Zhao G, Li C, Balluz LS (2013) Has the use of complementary and alternative medicine therapies by U.S. adults with chronic disease-related functional limitations changed from 2002 to 2007? J Altern Complement Med 19: 217-223.
- Barnes PM, Powell-Griner E, McFann K, Nahin RL (2004) Complementary and alternative medicine use among adults: United States, 2002. Adv Data: 1-19.
- Nolkemper S, Reichling J, Stintzing FC, Carle R, Schnitzler P (2006) Antiviral effect of aqueous extracts from species of the Lamiaceae family against Herpes simplex virus type 1 and type 2 in vitro. Planta Med 72: 1378-1382.
- HeidaryNavid M, Laszczyk-Lauer MN, Reichling J, Schnitzler P (2014) Pentacyclictriterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 21: 1273-1280.
- Jarikasem S, Charuwichitratana S, Siritantikorn S, Chantratita W, Iskander M, et al. (2013) Antiherpetic Effects of Gynuraprocumbens. Evid Based Complement Alternat Med 2013: 394-865.
- Reichling J, Koch C, Stahl-Biskup E, Sojka C, Schnitzler P (2005) Virucidal activity of a beta-triketone-rich essential oil of Leptospermum scoparium (manuka oil) against HSV-1 and HSV-2 in cell culture. Planta Med 71: 1123-1127.
- Carson CF, Hammer KA, Riley TV (2006) Melaleucaalternifolia (Tea Tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev 19: 50-62.
- Farag RS, Shalaby AS, El-Baroty GA, Ibrahim NA, Ali MA, et al. (2004) Chemical and biological evaluation of the essential oils of different Melaleuca species. Phytother Res 18: 30-35.
- Schnitzler P, Schon K, Reichling J (2001) Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Pharmazie 56: 343-347.
- Schnitzler P, Koch C, Reichling J (2007) Susceptibility of drug-resistant clinical herpes simplex virus type 1 strains to essential oils of ginger, thyme, hyssop, and sandalwood. Antimicrob Agents Chemother 51: 1859-1862.
- Nolkemper S, Reichling J, Sensch KH, Schnitzler P (2010) Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine 17: 132-138.
- Schnitzler P, Neuner A, Nolkemper S, Zundel C, Nowack H, et al. (2010) Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res 24 Suppl 1: S20-28.
- Schnitzler P, Schuhmacher A, Astani A, Reichling J (2008) Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomedicine 15: 734-740.
- Astani A, Navid MH, Schnitzler P (2014) Attachment and penetration of acyclovir-resistant herpes simplex virus are inhibited by Melissa officinalis extract. Phytother Res 28: 1547-1552.
- Wolbling RH, Leonhardt K (1994) Local therapy of herpes simplex with dried extract from Melissa officinalis. Phytomedicine 1: 25-31.
- Herold BC, Visalli RJ, Susmarski N, Brandt CR, Spear PG (1994) Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparansulphate and glycoprotein B. J Gen Virol 75: 1211-1222.
- Spear PG, Shieh MT, Herold BC, WuDunn D, Koshy TI (1992) Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv Exp Med Biol 313: 341-353.
- Mardberg K, Trybala E, Tufaro F, Bergstrom T (2002) Herpes simplex virus type 1 glycoprotein C is necessary for efficient infection of chondroitin sulfate-expressing gro2C cells. J Gen Virol 83: 291-300.
- MacLeod DT, Nakatsuji T, Yamasaki K, Kobzik L, Gallo RL (2013) HSV-1 exploits the innate immune scavenger receptor MARCO to enhance epithelial adsorption and infection. Nat Commun 4: 1963.
- Montgomery RI, Warner MS, Lum BJ, Spear PG (1996) Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87: 427-436.
- Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG (1998) Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280: 1618-1620.
- Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, et al. (1999) A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99: 13-22.
- Cocchi F, Fusco D, Menotti L, Gianni T, Eisenberg RJ, et al. (2004) The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. ProcNatlAcadSci USA 101: 7445-7450.
- Krummenacher C, Supekar VM, Whitbeck JC, Lazear E, Connolly SA, et al. (2005) Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J 24: 4144-4153.
- Nicola AV, McEvoy AM, Straus SE (2003) Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol 77: 5324-5332.
- Nicola AV, Straus SE (2004) Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol 78: 7508-7517.
- WuDunn D, Spear PG (1989) Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol 63: 52-58.
- Davis-Poynter N, Bell S, Minson T, Browne H (1994) Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J Virol 68: 7586-7590.
- Johnson DC, Frame MC, Ligas MW, Cross AM, Stow ND (1988) Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol 62: 1347-1354.
- Nahmias AJ, Kibrick S (1964) Inhibitory effect of heparin on herpes simplex virus. J Bacteriol 87: 1060-1066.
- Geuenich S, Goffinet C, Venzke S, Nolkemper S, Baumann I, et al. (2008) Aqueous extracts from peppermint, sage and lemon balm leaves display potent anti-HIV-1 activity by increasing the virion density. Retrovirology 5: 27.
- Handler CG, Eisenberg RJ, Cohen GH (1996) Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol 70: 6067-6070.
- Banfield BW, Leduc Y, Esford L, Schubert K, Tufaro F (1995) Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J Virol 69: 3290-3298.
- Bender FC, Whitbeck JC, Lou H, Cohen GH, Eisenberg RJ (2005) Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J Virol 79: 11588-11597.
- Satoh T, Arii J, Suenaga T, Wang J, Kogure A, et al. (2008) PILRalpha is a herpes simplex virus-1 entry coreceptor that associates with glycoprotein B. Cell 132: 935-944.
- Arii J, Goto H, Suenaga T, Oyama M, Kozuka-Hata H, et al. (2010) Non-muscle myosin IIA is a functional entry receptor for herpes simplex virus-1. Nature 467: 859-862.
- Suenaga T, Satoh T, Somboonthum P, Kawaguchi Y, Mori Y, et al. (2010) Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. ProcNatlAcadSci USA 107: 866-871.
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778-790.
- Bengali Z, Townsley AC, Moss B (2009) Vaccinia virus strain differences in cell attachment and entry. Virology 389: 132-140.
- Conti C, Mastromarino P, Riccioli A, Orsi N (1991) Electrostatic interactions in the early events of VSV infection. Res Virol 142: 17-24.
- Sharma A, Li X, Bangari DS, Mittal SK (2009) Adenovirus receptors and their implications in gene delivery. Virus Res 143: 184-194.
- Dechecchi MC, Melotti P, Bonizzato A, Santacatterina M, Chilosi M, et al. (2001) Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J Virol 75: 8772-8780.
- Ewers H, Romer W, Smith AE, Bacia K, Dmitrieff S, et al. (2010) GM1 structure determines SV40-induced membrane invagination and infection. Nat Cell Biol, pp: 11-12.
- Tavakkol A, Burness AT (1990) Evidence for a direct role for sialic acid in the attachment of encephalomyocarditis virus to human erythrocytes. Biochemistry 29: 10684-10690.
- Guy M, Chilmonczyk S, Cruciere C, Eloit M, Bakkali-Kassimi L (2009) Efficient infection of buffalo rat liver-resistant cells by encephalomyocarditis virus requires binding to cell surface sialic acids. J Gen Virol 90: 187-196.
- Reiter DM, Frierson JM, Halvorson EE, Kobayashi T, Dermody TS, et al. (2011) Crystal structure of reovirus attachment protein sigma1 in complex with sialylated oligosaccharides. PLoSPathog 7: e1002166.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences